Photodynamic therapy for esophageal cancer
What is photodynamic therapy (PDT)?
PDT is a laser treatment involving photosensitizer, which is a photosensitive molecule such as porphyrin, and light of a specific wavelength. The photosensitizer is administrated via an oral or intravenous route, and is localized to a target tumor cell; then, light of a specific wavelength activates the sensitizer (1). This photodynamic reaction induces a chemical destruction of the tumor tissue mediated by singlet molecular oxygen and other reactions. Damage to the tissue occurs through several pathways, including cell necrosis, apoptosis, and ischemia with vascular shutdown (2). The most popular photosensitizer is porphyrin, and PDT is an effective treatment and is being tested to treat many cancers, such as those of the skin, head and neck, brain, lung, bladder, gastrointestinal (GI) tract, and others.
Present state of PDT for esophageal cancer
In Japan, PDT using porfimer sodium (Photofrin, Pfizer Japan Inc., Japan) followed by 630 nm wavelength excimer dye laser irradiation (EDL-1 or 2, Hamamatsu Photonics, Hamamatsu, Japan) is approved for early stage of lung cancer, esophageal cancer, gastric cancer, and cervical cancer. Between September 1990 and March 1992, a clinical trial of PDT for patients with superficial esophageal cancer was conducted, and 9 of 10 patients achieved complete response (CR) (CR rate: 90%) (3). Because of the favourable results of this study, PDT using porfimer sodium was approved as a curative treatment for superficial esophageal cancer in 1994.
PDT procedures using porfimer sodium commence with the intravenous administration of 2 mg/kg of Photofrin. Subsequently, laser treatment using the 630 nm wavelength excimer dye laser is performed 48-72 hours after drug administration. The excimer dye laser is delivered via a microlens fiber through the operative channel of the endoscope, and was positioned in front of the lesions. The distal tip of the fiber is maintained to keep approximately 1 cm from the lesion, and laser is irradiated using total light density of 60-150 J/cm2 with a maximum pulse energy of 4 mJ per pulse and a 40 Hz pulse frequency. If the lesions are large, laser irradiation is performed on overlapping sections as the Olympic symbol. The manufacturer recommends the use of porfimer sodium as PDT for esophageal cancer lesions with the following characteristics: (I) smaller than half of the circumference of the lumen and 2 cm in diameter; (II) limited to within the submucosal layer in depth; and (III) judged as difficult to remove with endoscopic resection.
Endoscopic submucosal dissection (ESD) was developed initially in gastric cancer to improve the curability of endoscopic resection for large lesions. However, use of the ESD procedure for superficial esophageal cancer has dramatically spread in Japan recently; therefore, the number of superficial lesions that are considered to be difficult to remove with ESD, and indicated for PDT has decreased. Although PDT for superficial esophageal cancer is not popular in Japan, some investigators have reported the results of using PDT for patients with superficial esophageal squamous cell carcinoma (ESCC). Nakamura et al. reported the results of PDT for seven patients with relatively small lesions (5-30 mm), all lesions were cured with no recurrence or severe complications (4). Tanaka et al. reported the results of PDT used against wide-spread lesions for which curative resection was considered to be difficult even with ESD (5). They treated 38 patients with superficial ESCC (31 lesions of T1a and 7 lesions of T1b without lymph node metastasis), and complete remission was achieved in 33 (87%) patients with PDT. There was no major complication and treatment related death, and at the median follow up period of 64 months, the 5-year survival rate was 76%. They concluded that PDT could be a possible curative treatment option for large superficial ESCC.
In contrast, palliative treatment for obstructive advanced esophageal cancer and curative treatment for precancerous lesions in Barrett’s esophagus are the major indications for PDT in US and European countries. Litle reported the treatment results of palliative PDT for 215 patients with symptomatic advanced or recurrent esophageal cancer, and approximately 85% of patients improved their dysphagia (6). They achieved a median of 2 months of dysphagia-free survival, and 4.8 months of overall survival (OS) time. The major complications were perforation (2.3%), photosensitivity (6%), and aspiration pneumonia (1.8%). Lindenmann et al. published a retrospective analysis of 171 patients who received multimodal palliative treatment for inoperable esophageal cancer and who achieved sufficient dysphagia relief and improved survival (7). They concluded that PDT could be a beneficial initial treatment for patients with inoperable advanced cancer without gross infiltration into other organs.
Furthermore, several reports of PDT for high grade dysplasia of Barrett’s esophagus were published, and a sufficient eradication rate of dysphagia was confirmed using not only porfimer sodium, but also aminolaevulinic acid (ALA) (8,9). Advantages of ALA compared with porfimer sodium were a shorter period of photosensitivity and a lower rate of esophageal structures when used for short segments of Barrett’s esophagus. ALA is a natural amino acid and a pro-drug of protoporphyrin IX; it has been used as a photosensitizer mainly in European countries.
PDT for local failure after chemoradiotherapy (CRT)
CRT is one of the curative treatment options for ESCC, even at an advanced stage. However, local failure without distant metastasis after completion of CRT remains a major problem that must be overcome to achieve a cure. Although salvage esophagectomy is now indicated for such patients, it has a higher morbidity and mortality rate compared with primary or planned esophagectomy (10-12). In addition, lymph node recurrence within the radiation field is negligible, if the local is under controlled (13). The development of curative and safe salvage treatment options for local failure is needed to improve the survival of patients treated with CRT. Although we have reported that endoscopic resection could be a curative salvage treatment option for carefully selected patients with local failure, the indication was limited to only for tiny local failure lesions (14). We considered that PDT could be more powerful procedure compared with endoscopic treatment, because PDT is indicated not only superficial cancer, but also palliative treatment for advanced esophageal cancer as described earlier.
We have introduced PDT as a salvage treatment for local failure after CRT, and reported that acceptable short term results could be achieved (15). The indication criteria of salvage PDT were determined to be the follows: (I) absence of lymph node or distant metastases by computed tomography (CT) before PDT; (II) residual or recurrent tumor at the primary site with a stage limited to within uT2 by endoscopic ultrasound (EUS); (III) endoscopic resection of ESD was not indicated for reasons of either concomitant deep ulceration or severe fibrosis due to radiation or lesion invading to the deep submucosal layer; and (IV) patient refusal of surgery or physical complications that would have made surgery intolerable. A CR was achieved in 22 of 37 patients (CR rate: 59.5%) after PDT (Figure 1). Moreover, the 5-year progression free survival (PFS) and OS were 20.7% and 36.1%, respectively (16).
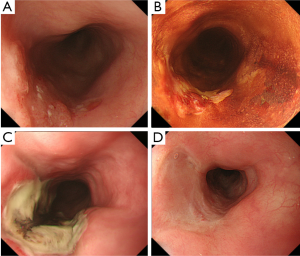
Subsequently, we conducted a prospective study to confirm the efficacy and safety of salvage PDT for local failure after CRT. A total of 25 patients with local failure limited to within the submucosal layer were enrolled, and a CR was attained in 19 patients with PDT (CR rate, 76%; 95% CI, 55-91%). One treatment related death (4%) was experienced caused by GI bleeding that was suspected to be due to an esophago-aortic fistula at the irradiated site approximately one month after PDT. With the median follow up period of approximately three years, the PFS and OS at three years were approximately 40%, and we concluded that PDT could be a curative option as salvage treatment for carefully selected patients without any metastasis.
PDT using next generation photosensitizer
First generation PDT using porfimer sodium has had some problems, such as a high risk of skin phototoxicity requiring a long sun shade period (4-6 weeks), and the need for a large and expensive excimer dye laser system. In fact, we have found that 34% of patients experience phototoxicity even with 2 weeks hospitalization and 8 weeks sun shade period (17). In contrast, talaporfin sodium (Laserphyrin for Injection, Meiji Seika Pharma Co., Ltd., Tokyo, Japan) is a second generation photosensitizer that is developed in Japan and featured as possessing more rapid clearance from the skin compared with porfimer sodium. Therefore, PDT using talaporfin sodium is expected to reduce phototoxicity even with a short sun shade period of two weeks or less. Furthermore, the diode laser system (PD laser, Panasonic Healthcare Co., Ltd., Ehime, Japan), which was also developed in Japan and emits 664 nm laser light to excite the talaporfin sodium, is a much smaller and less expensive system compared with the excimer dye laser system. PDT using talaporfin sodium and the diode laser system demonstrated a high response rate and similar efficacy compared with first generation PDT, with modest skin phototoxicity, in a clinical trial for early lung cancer (18). However, PDT using talaporfin sodium and the diode laser is confirmed of its efficacy and approved only for lung cancer and malignant brain tumors. While we had wanted to introduce this new combination of PDT for esophageal cancer, its safety and efficacy for this use had not yet been evaluated even in animal models. First, we have evaluated the tissue damage of a normal esophagus caused by photoactivation with talaporfin sodium and a diode laser in a living canine model (19). In this pre-clinical study, laser irradiation was escalated with three levels of fluence (25, 50, and 100 J/cm2) after administration of talaporfin sodium for three dogs at each level; the canine tissues were then evaluated one week after laser irradiation. From the results of the pathological evaluation we found that the tissue damage had worsened in a stepwise fashion at every increase in the fluence levels. Next, we conducted a phase I study to find the appropriate intensity of the diode laser for patients with local failure after CRT for esophageal cancer (20). In that study, no patient experienced severe adverse events or phototoxicity, and we established that 100 J/cm2 was the recommended fluence for treating local failure after CRT. The next generation PDT also demonstrated promising efficacy. From the results of this study, we are now conducting a multi-institutional phase II study of PDT using talaporfin sodium and a diode laser to acquire the approval for its use in the treatment of esophageal cancer in Japan.
Conclusions
While PDT is approved as a curative treatment for superficial esophageal cancer in Japan, it lost the popularity due to the dramatic spread of ESD. Recently, the advantages of PDT are being reconsidered after favorable results of salvage treatment in patients with local failure after CRT. Furthermore, photosensitizers that require only a short sun shade period have been developed, and promising results of PDT as a salvage treatment for esophageal cancer were observed in our study. If this new PDT is approved for esophageal cancer in Japan, salvage PDT may become a popular and effective option, and could contribute to increasing quality of life for esophageal cancer survivors through organ preservation.
Acknowledgements
Disclosure: The authors declare no conflict of interest.
References
- Dolmans DE, Fukumura D, Jain RK. Photodynamic therapy for cancer. Nat Rev Cancer 2003;3:380-7. [PubMed]
- Prosst RL, Wolfsen HC, Gahlen J. Photodynamic therapy for esophageal diseases: a clinical update. Endoscopy 2003;35:1059-68. [PubMed]
- Yoshida K, Suzuki S, Mimura S, et al. Photodynamic therapy for superficial esophageal cancer: a phase III study using PHE and excimer dye laser. Gan To Kagaku Ryoho 1993;20:2063-6. [PubMed]
- Nakamura T, Fukui H, Shirakawa K, et al. Photodynamic therapy of superficial esophageal cancer with a transparent hood. Gastrointest Endosc 2004;60:120-4. [PubMed]
- Tanaka T, Matono S, Nagano T, et al. Photodynamic therapy for large superficial squamous cell carcinoma of the esophagus. Gastrointest Endosc 2011;73:1-6. [PubMed]
- Litle VR, Luketich JD, Christie NA, et al. Photodynamic therapy as palliation for esophageal cancer: experience in 215 patients. Ann Thorac Surg 2003;76:1687-92; discussion 1692-3.
- Lindenmann J, Matzi V, Neuboeck N, et al. Individualized, multimodal palliative treatment of inoperable esophageal cancer: clinical impact of photodynamic therapy resulting in prolonged survival. Lasers Surg Med 2012;44:189-98. [PubMed]
- Gossner L, Stolte M, Sroka R, et al. Photodynamic ablation of high-grade dysplasia and early cancer in Barrett’s esophagus by means of 5-aminolevulinic acid. Gastroenterology 1998;114:448-55. [PubMed]
- May A, Gossner L, Pech O, et al. Intraepithelial high-grade neoplasia and early adenocarcinoma in short-segment Barrett’s esophagus (SSBE): curative treatment using local endoscopic treatment techniques. Endoscopy 2002;34:604-10. [PubMed]
- Swisher SG, Wynn P, Putnam JB, et al. Salvage esophagectomy for recurrent tumors after definitive chemotherapy and radiotherapy. J Thorac Cardiovasc Surg 2002;123:175-83. [PubMed]
- Miyata H, Yamasaki M, Takiguchi S, et al. Salvage esophagectomy after definitive chemoradiotherapy for thoracic esophageal cancer. J Surg Oncol 2009;100:442-6. [PubMed]
- Tachimori Y, Kanamori N, Uemura N, et al. Salvage esophagectomy after high-dose chemoradiotherapy for esophageal squamous cell carcinoma. J Thorac Cardiovasc Surg 2009;137:49-54. [PubMed]
- Onozawa M, Nihei K, Ishikura S, et al. Elective nodal irradiation (ENI) in definitive chemoradiotherapy (CRT) for squamous cell carcinoma of the thoracic esophagus. Radiother Oncol 2009;92:266-9. [PubMed]
- Yano T, Muto M, Hattori S, et al. Long-term results of salvage endoscopic mucosal resection in patients with local failure after definitive chemoradiotherapy for esophageal squamous cell carcinoma. Endoscopy 2008;40:717-21. [PubMed]
- Yano T, Muto M, Minashi K, et al. Photodynamic therapy as salvage treatment for local failures after definitive chemoradiotherapy for esophageal cancer. Gastrointest Endosc 2005;62:31-6. [PubMed]
- Yano T, Muto M, Minashi K, et al. Long-term results of salvage photodynamic therapy for patients with local failure after chemoradiotherapy for esophageal squamous cell carcinoma. Endoscopy 2011;43:657-63. [PubMed]
- Yano T, Muto M, Minashi K, et al. Photodynamic therapy as salvage treatment for local failure after chemoradiotherapy in patients with esophageal squamous cell carcinoma: a phase II study. Int J Cancer 2012;131:1228-34. [PubMed]
- Kato H, Furukawa K, Sato M, et al. Phase II clinical study of photodynamic therapy using mono-L-aspartyl chlorin e6 and diode laser for early superficial squamous cell carcinoma of the lung. Lung Cancer 2003;42:103-11. [PubMed]
- Horimatsu T, Muto M, Yoda Y, et al. Tissue damage in the canine normal esophagus by photoactivation with talaporfin sodium (laserphyrin): a preclinical study. PLoS One 2012;7:e38308. [PubMed]
- Yano T, Muto M, Yoshimura K, et al. Phase I study of photodynamic therapy using talaporfin sodium and diode laser for local failure after chemoradiotherapy for esophageal cancer. Radiat Oncol 2012;7:113. [PubMed]


