Phase II trial of temozolomide and reirradiation using conformal 3D-radiotherapy in recurrent brain gliomas
Introduction
Recurrent brain glioma is an incurable disease. The great majority of malignant gliomas (at least 70%) recur after initial treatment (1).
There are several factors associated with recurrence including pathologic subtypes as patients with high grade glioma (grade III, IV) tend to recur more often than other subtypes of gliomas and patients with anaplastic histologic features (number of mitoses, high labeling index, endothelial proliferation, nuclear pleomorphism, and Necrosis) tend to recur more than patients without these features (1,2).
Also, there are some genetic abnormalities linked with recurrence including deletion of CDKN2A, deletion of P14ARF, amplification/overexpression of the CDK4 gene, and EGFR amplification/overexpression or PGFR overexpression (3).
There is no standard treatment for recurrent brain glioma. The prognosis of recurrent brain glioma is very poor with few breakthroughs in management over the past few decades. Patients without treatment usually survive for few months (4).
Temozolomide as an imidazotetrazine agent is effective in recurrent glioma. Myelotoxicity (primarily neutropenia and thrombocytopenia) is the major adverse effect of temozolomide observed in number of clinical trials (5,6).
The brain tolerance for reirradiation depends on several factors including dose per fraction, total dose administered, overall treatment time, time interval between primary treatment and reirradiation, volume of brain irradiated, adjunctive therapies, and other factors (7,8).
A wide variety of radiotherapy (RT) techniques have been used for reirradiation including 3D-conformal RT, intensity-modulated radiotherapy (IMRT), brachytherapy, stereotactic radiosurgery (SRS), fractionated stereotactic radiotherapy (FSRT), and hypofractionated stereotactic radiotherapy (H-FSRT) (8).
This study commenced in Cairo, Egypt, in 2006 as a prospective, open-label study in patients with recurrent malignant glioma.
This phase II trial has two end points. The primary end point was response, and the secondary end points included overall survival (OS), progression-free survival (PFS), the quality of life (QOL), and toxicity.
Patients and methods
Patients with high grade glioma (grade III, and IV) were eligible for the study if they had unequivocal evidence of tumour recurrence as shown by gadolinium-enhanced magnetic resonance imaging (MRI) after failing conventional RT, with or without chemotherapy for initial disease.
Inclusion criteria included different subtypes of histological proven high brain glioma, Karnofsky performance status (KPS) ≥60, and aged above 18 and below 70 years. Patients were also required to have adequate haematological [WBC >3,000/mm3, absolute neutrophil count (ANC) ≥1,500/mm3, platelets ≥100,000/mm3, Hemoglobin >9 gm/dL], renal (Creatinine ≤1.5 mg/dL), and liver functions (total Bilirubin ≤1.5 mg/dL, transaminases ≤2 times above the institutional upper normal limit). ALL patients must have received RT as part of their initial therapy. An interval of at least 3 months must have elapsed since the completion of the most recent course of radiation therapy, while at least 3 weeks must have elapsed since the completion of a non-nitrosourea containing chemotherapy regimen and at least 6 weeks since the completion of a nitrosourea containing chemotherapy regimen. Female patients of childbearing potential must have a negative pregnancy test (serum b-HCG); and both male and female patients must employ effective contraceptive measures prior to start of therapy until four weeks after the last dose of study drug.
Additionally, patients must have no concurrent malignancy.
The study was conducted in Oncology Unit, Ain Shams University Specialist Hospital (ASUSH) in the period between February 2004 and December 2008. All patients should sign the consent of clinical trial enrolment before inclusion into the trial.
Study design
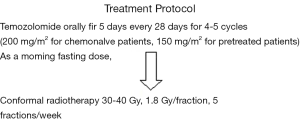
The study design is shown in (Figure 1).
While on temozolomide, patients were advised to receive prophylaxis against pneumocystis carinii pneumonia (PCP) especially in the first two cycles, and antiemetic prophylaxis from day 1 to day 10 of each cycle.
Reiradiation was done using 3D-conformal RT.
Technique of reirradiation
Patient positioning and immobilization
Supine with neutral neck position. Immobilization was maintained with the use of the commercially available thermoplastic devices.
Target localization
Target volume was defined by MRI data in corporation with treatment planning CT.
Volumes definition
T1-images on MRI were used to define the Gross tumor volume (GTV). T2-weighted and FLAIR images were used to define the clinical target volume (CTV). The planning target volume (PTV) was defined by adding 1 cm to the GTV to include the surrounding oedema. The PVT was reduced in areas near organ at risks (OARs). These OARs included the eyes, optic nerves, optic chiasm, and brainstem. The beams directions, portals were defined for adequate and homogenous coverage of the target volume. The target was considered to be appropriately treated if the PTV was enclosed within the 95% to 105% isodose line. During RT, patients were kept on dexamethasone 4 mg orally, every 8 hours, with PPI 40 mg orally, once daily, and antiepileptic prophylaxis.
Evaluation
Baseline neurological clinical examination and MRI were performed before treatment. During treatment protocol, patients were closely followed twice weekly for new complaints or treatment related complications. Before subsequent cycles of chemotherapy, patients were required to repeat CBC, blood chemistry tests, and a physical examination. In addition, after chemotherapy (before RT), patients were required to repeat neuroimaging. Also, before RT, patients were required to repeat CBC, blood chemistry tests, and a physical examination. After treatment protocol, patients were followed up regularly as routine follow up visits every month for the first six months then every 3 months thereafter.
Patients were evaluated after treatment protocol for both subjective and objective response (Table 1) by history taking, physical examination, laboratory investigations that include CBC, blood chemistry tests, and MRI brain. MRI brain (or CT if MRI was medically contraindicated) was done after chemotherapy cycles, after treatment protocol, and every 2 months in first 6 months, then every 3 months thereafter for follow up.
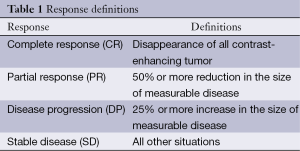
Full table
QOL evaluation
The trial applied FACT/NCCN (The Functional Assessment of Cancer Therapy/National Cancer comprehensive network)-Brain Symptom Index (FBrSI)-15 questionnaires to assess the QOL.
The FBrSI-15 questionnaires (after translation to Arabic) were asked to patients in four occasions; at start of treatment (baseline), after the end of treatment protocol, 4 weeks after treatment, and 4 weeks thereafter.
Improved QOL scores were defined as clinically significant if a change of the score of 4 points or more.
Toxicity
Toxic effects were graded according to the National Cancer Institute Common Toxicity Criteria, version 2.0.
Repeat cycles of chemotherapy were administered on schedule only if the patient met the following re-treatment criteria:
ANC ≥1,500/mm3, platelets ≥100,000/mm3, Haemoglobin >9 gm/dL, Creatinine ≤1.5 mg/dL, total bilirubin ≤1.5 mg/dL, transaminases ≤2 times above the institutional upper normal limit, and all other toxicities must have resolved to baseline or grade 1.
Statistical analysis
The objective response rate was calculated by pooling the CR, and PR rates. Mean and median values were used for the description of continuous data. OS and PFS were analyzed by the Kaplan-Meier method, with use of two-sided log-rank statistics. P value was calculated by fisher’s exact test. P value was significant at ≤0.05 levels, highly significant at ≤0.01, and insignificant at >0.05. All calculations were carried out using SPSS (Statistical Package for Social Sciences) software for windows, version 15 (SPCC Inc., Chicago, USA).
Results
Between February 2006 and June 2009, 29 patients (20 males, and 9 females) with recurrent brain glioma were recruited. All had unequivocal radiological evidence of recurrence. The mean age was 42.1 years (range, 18-65 years). The mean KPS was 78.8. There were 20 patients with recurrent GBM, and 9 with AA. Twenty-eight patients presented by localized recurrence. Only 1 patient presented with diffuse recurrence. Of those 28 patients, 16 had temporal lobe lesions, 9 had parietal lesions, 1 had frontal lesions, 1 had occipital lesion, and 1 had cerebellar lesion. The mean tumour size of the study group was 3.7 cm (range, 1-7 cm) (Table 2). All the 29 patients had received RT treatment in the management of their primary lesion at a dose range of 55-70 Gy. Ten patients received previous chemotherapy, 4 PCV, and 6 temozolomide. The time interval between the two RT courses (primary and reirradiation) was in the range of 8 to 18 months, with a mean of 12.8 months, and a median of 13 months.
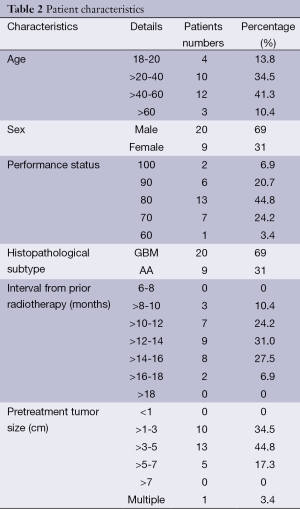
Full table
All patients underwent the treatment protocol. The main cycles of temozolomide were 4.5 (range, 4-5 cycles). Patients were given reirradiation after the end of the last chemotherapy cycle by mean time of 2.5 weeks (range, 2-3 weeks). The main RT dose delivered was 36 Gy (range, 30-40 Gy).
Response data
The response was checked by MRI that was done 2.3 weeks (range, 2-3 weeks) after finish of reirradiation. Objective Responses (CR, PR) were observed in 6 out of 29 patients (Table 3). Two patients achieved CR (two patients with GBM). Four patients achieved PR (three with AA, and one with GBM), with an overall objective Response rate of the study of 20.6%. SD was achieved in ten patients (four patients with GBM, six with AA), with a SD rate of the study was 34.4%. DP was observed in the remaining 13 patients with DP rate of the study was 45%.
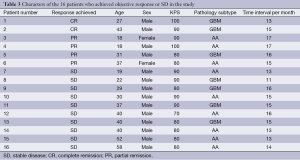
Full table
Survival data
The mean PFS of the study group was 10.1 months (range, 4-22+ months). The median PFS was 10 months. The mean OS was 11.4 months (range, 4-22+ months). The median OS was 11 months.
The 6-month PFS was 47%. The 6-month OS was 73% for the study group.
QOL data
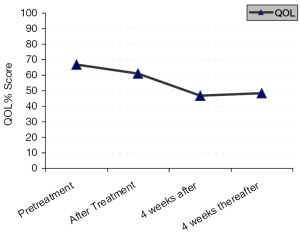
After analysis of QOL using FACT/NCCN BRAIN SYMPTOM INDEX (FBrSI)-15 questionnaires, we observed before treatment (at baseline) that, (after assessment of 23 patients who respond to the questionaires), (Figure 2) the mean score was 40/60 (66.7%), (range, 34/60 to 44/60) (73.3-56.7%).
Just after treatment (after assessment of the 23 patients), the mean score was 36.7/60 (61%), (range, 30/60 to 39/60) (50-65%),
At 4 weeks after treatment (after assessment of the 17 patients who achieved objective response or SD), the mean score was 28/60 (46.7%), (range, 21/60 to 39/60) (35-65%). At 4 weeks thereafter (after assessment of 15 out of the 17 patients), the mean score was 29/60 (48.3%), range, 21/60 to 35/60 (35-58%).
Case presentation
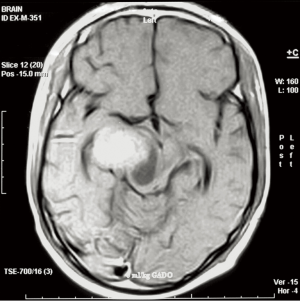
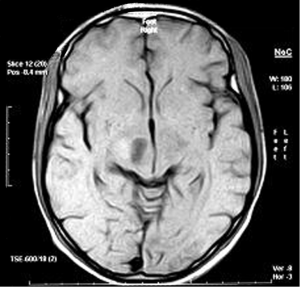
Eighteen years old male with recurrent AA dated on October 2006, underwent four cycles of temozolomide, followed by conformal RT, and achieved PR. Following treatment, he maintained in PR for 13 months, with a PFS for 19 months from recurrence.
MRI before treatment protocol (Figure 3).
MRI after treatment protocol showing PR (Figure 4).
Toxicity profile
Toxicities were evaluated in all the 29 patients enrolled in the study. Chemotherapy related side effects: of 125 chemotherapy cycles given, grade 1 or 2 toxicity haematological toxicity occurred in 12 cycles (8.6%):
- -Grade 1 or 2 neutropenia was observed in five cycles;
- -Grade 1 or 2 thrombocytopenia was observed in seven cycles;
- -No patient required dose reduction or cycle delay.
Other non-hematologic toxicities
Grade 1 or 2 toxicity in the form of nausea and vomiting occurred in 50/125 of cycles (40%).
Radiotherapy related side effects
Among the 29 patients treated by conformal 3D-reirradiation, no acute neurologic toxicity of grade II or higher was observed. Only, minor temporary acute radiation side effects included alopecia, headaches, grade 1, 2 nausea/vomiting responded to antiemetics, and skin erythema. There was no late radiation morbidity observed in the follow-up period.
Discussion
Recurrent brain glioma is an incurable disease. Treatment options for recurrent brain glioma include chemotherapy, radiation therapy, surgery, and palliation (9).
One of the main issues in treating recurrent high-grade brain glioma is the principal decision if a definitive therapy is appropriate or just palliative care. Another issue is that the best treatment regimen. The main goal of surgery is removal of the enhancing tissues to decrease pressure effect and to provide diagnosis in ambiguous cases (9).
Temozolomide as an imidazotetrazine agent is effective in recurrent glioma (6).
Temozolomide appears effective in patients with recurrent high grade gliomas. It is associated with objective response rate of 35% in AA, and 11% in GBM. It achieves 6-month PFS of 46% in AA, and 29% in GBM (10-12).
In the current study, 29 patients with recurrent brain glioma were treated by combined modality using temozolomide for 4-5 cycles, then conformal RT. Most of the clinical researchers prefer to give temozolomide alone instead of chemoradiation in recurrent brain glioma that may be attributed to:
- -Toxicity of combined modality is expected to be much higher than each modality. However, from literature reviews, when comparing the rate of severe toxicity (grade 3, 4) by temozolomide alone with that of temozolomide plus reirradiation, it was in the range of 6-18%, and 11-19% respectively.
- -Fear of giving two irradiation treatment courses in short time interval (<12 months), due to insufficient data that cover this point of interest (13-15).
When reviewing trials published within the last 6 years in recurrent brain glioma, it was found that in order to achieve a good objective response, and survival benefit, researchers used either temozolomide for median of eight cycles alone, or temozolomide for median of 4-6 cycles plus other agents including RT (15).
However, giving temozolomide beyond six cycles may be associated with more toxicities as those shown in the study by Van den Bent et al., 2001(16), who used TZM for median of ten cycles. They observed the following toxicity profiles:
- -Dose reduction and delay of treatment in 26% of cycles;
- -Grade 3 or 4 hematologic toxicity in 4% of cycles;
- -Grade 3/4 nausea and/or vomiting in 1% of cycles, and;
- -One patient developed a grade 4 febrile neutropenia.
It was avoided to give temozolomide concurrently with RT, in order to avoid unexpected toxicities in those previously irradiated patients.
In the current study, 29 Egyptian patients showed objective response rate, and survival benefit comparable with other studies done in Caucasian patients (17-19).
The current study included patients with AA, and GBM. When evaluating the response data in AA, the current treatment protocol achieved ORR of 33%, and SD in the remaining 66.6% of patients. In GBM, the current treatment protocol achieved ORR of 15%, and SD in 15% of patients. The 6-month PFS was 65%. The 6-month OS was 83% for AA group. The 6-month PFS was 39%. The 6-month OS was 69% for GBM group. The 6-month PFS was 47%.
In the current study, the QOL data were analyzed as important information about the response to treatment. A subjective response is considered important factor for treatment response, and sometimes as important as the objective response in recurrent brain glioma. One of the aims of treatment of such cancers is to allow patients enjoy their life without significant neurological deterioration, seizures, or psychological troubles. The current treatment protocol significantly improved the QOL in patients with recurrent brain glioma, from a mean score of 40/60 (66.7%) before treatment, to a mean score of 28/60 (46.7%) 4 weeks after treatment, and a mean score of 29/60 (48.3%) 4 weeks thereafter.
However, when assessing the QOL at the end of treatment, there were non-significant changes. (From mean of 40/60 to 36.7/60), the difference in between the 2 scores is less than 4 (i.e., non-clinically significant). This could be related to treatment related side effects.
The current treatment protocol was well tolerated, with only mild side effects. Temozolomide had demonstrated a safe drug profile in the current study, with mild emetogenic effects during the days of drug ingestion (days 1-5), and was relieved by anti-emetics. The drug had reversible myelosuppression (neutropenia, and thrombocytoprnia). Nadir platelet and neutrophil counts typically occurred on days 21 to 26 of the cycles. No grade 3 or 4 toxicity was recorded.
Reirradiation showed no acute neurologic toxicity of grade II or higher. Only observed, minor (grade 1) temporary acute radiation side effects included headaches, mild (grade 1, 2) nausea/vomiting responded to antiemetics, and skin erythema. There was no late radiation morbidity observed.
Acknowledgements
Disclosure: The author declares no conflict of interest.
References
- Brandes AA, Vastola F, Monfardini S. Reoperation in recurrent high-grade gliomas: literature review of prognostic factors and outcome. Am J Clin Oncol 1999;22:387-90. [PubMed]
- Melissa LB, El-Zein R, Scheurer ME. Epidemiology of brain tumors, Chapter1. In Tumors of the brain and spine. M.D. Anderson Cancer Care Series. eds. DeMonte F, Gilbert MR, Mahajan A, et al. Springer Science Business Media, USA, 2007.
- van den Boom J, Wolter M, Kuick R, et al. Characterization of gene expression profiles associated with glioma progression using oligonucleotide-based microarray analysis and real-time reverse transcription-polymerase chain reaction. Am J Pathol 2003;163:1033-43. [PubMed]
- Dillon A. Guidance on the use of temozolomide for the treatment of recurrent malignant glioma. (brain cancer). NICE Technology Appraisal Guidance – No.23, 2001. National Institute for Clinical Excellence, UK.
- Osoba D, Brada M, Yung W. Temozolomide May Improve the Outlook for Patients with Recurrent Malignant Gliomas. Drug Ther Perspect 2000;15:1-5.
- Yung A, Levin VA, Albright R, et al. Randomized trial of Temodal vs. procarbazine in glioblastoma multiforme at first relapse [abstract 532]. 35th Proc Am Soc Clin Oncol 1999;18:139a.
- Schultheiss TE, Kun LE, Ang KK, et al. Radiation response of the central nervous system. Int J Radiat Oncol Biol Phys 1995;31:1093-112. [PubMed]
- Veninga T, Langendijk HA, Slotman BJ, et al. Reirradiation of primary brain tumours: survival, clinical response and prognostic factors. Radiother Oncol 2001;59:127-37. [PubMed]
- Hentschel SJ, Sawaya R. Optimizing outcomes with maximal surgical resection of malignant gliomas. Cancer Control 2003;10:109-14. [PubMed]
- Newlands ES. Temozolomide May Improve the Outlook for Patients With Recurrent Malignant Gliomas. Drug Ther Perspect 2000;15:1-5.
- Prados M, Yung A, Chang S, et al. A phase II trial of Temodal® (temozolomide) in patients with anaplastic astrocytoma at first relapse [abstract 533]. 35th Proc Am Soc Clin Oncol 1999;18:139a.
- Yung WK, Albright RE, Olson J, et al. A phase II study of temozolomide vs. procarbazine in patients with glioblastoma multiforme at first relapse. Br J Cancer 2000;83:588-93. [PubMed]
- Cassano A, Longo R, Angelelli L, et al. Temozolomide in First Line Treatment of Recurrent or Residual Malignant Brain Tumors. ASCO Proceedings, Annual Meeting, www.asco.com. 2001: Abstract No. 2058.
- Neyns B, Everaert E, Joosens E, et al. Temozolomide for the treatment of recurrent glioma: Results of a compassionate use program in Belgium. J Clin Oncol 2004;22:abstr 1541.
- Prados MD, Yung WK, Fine HA, et al. Phase 2 study of BCNU and temozolomide for recurrent glioblastoma multiforme: North American Brain Tumor Consortium study. Neuro Oncol 2004;6:33-7. [PubMed]
- van den Bent MJ, Keime-Guibert F, Brandes AA, et al. Temozolomide chemotherapy in recurrent oligodendroglioma. Neurology 2001;57:340-2. [PubMed]
- Nieder C, Wiedenmann N, Stark S, et al. Phase II trial of functional imaging-optimized stereotactic fractionated radiotherapy plus temozolomide for recurrent high-grade glioma. ASCO Annual Meeting. Abstract No: 1552, 2004.
- Quinn JA, Reardon DA, Friedman AH, et al. Phase II trial of temozolomide in patients with progressive low-grade glioma. J Clin Oncol 2003;21:646-51. [PubMed]
- Schönekaes K, Mücke R, Panke J, et al. Combined radiotherapy and temozolomide in patients with recurrent high grade glioma. Tumori 2002;88:28-31. [PubMed]