Impact of metformin on reproductive tissues: an overview from gametogenesis to gestation
Introduction
Metformin is the most widely used drug for reproductive abnormalities associated with insulin resistance and also the oldest insulin sensitizer in the therapeutic management of type 2 diabetes mellitus. Its action reduces hepatic glucose output, increases tissue insulin sensitivity and enhances peripheral glucose uptake, resulting in lower concentrations of glucose without the associated risk of either hypoglycemia or weight gain (1,2). Metformin is a stable hydrophilic biguanide compound that is highly polar, positively charged with a low molecular weight and has pleiotropic actions. It is present in a number of tissues including muscle, liver, pancreas, adipose tissue, hypothalamus, pituitary and the gonads. Despite low lipid solubility, some subcellular studies in rat liver showed that metformin is mainly localized in the cytosol (3) and studies in mice show that metformin may accumulate in certain tissues at higher concentrations than in plasma (4). The passive diffusion of metformin into cells is limited (5), the main transport is the organic cation transporter 1-3 or multidrug and toxic compound extrusion type transporters (MATE1, MATE2) which are able to internalize metformin as described in gut, hepatocytes, renal tubular epithelial cells and reproductive tissues (6). One of the direct effects of metformin identified is to inhibit the activity of the respiratory electron transport chain in mitochondria (7) and to activate the cytoplasmic protein kinase known as AMP-activated protein kinase (AMPK) (8). AMPK is an important sensor of cellular energy homeostasis and is sensitive to the AMP:ATP ratio (9,10). We can note that several studies have also demonstrated that metformin might act independently of AMPK (11-13). A deficiency in ATP activates AMPK leading to increased energy production including glucose and lipid catabolism and an inhibition of energy consuming processes such as protein, fatty acid and cholesterol synthesis.
These metabolic pathways are highly utilised by reproductive tissues which produce steroids, peptides hormones, and where proliferative cells are present. Furthermore, metformin administration has been used in women who present with perturbed fertility associated with insulin resistance. Indeed, women with polycystic ovary syndrome (PCOS) were treated with clomiphene citrate associated or not with metformin to improve ovulation rate (14-16).
The aims of this review are to provide a critical summary of the safety of metformin on various aspects of sexual reproduction, especially the possible side-effects on gonads of offspring from women who are administered metformin during pregnancy.
Metformin and central control of reproduction
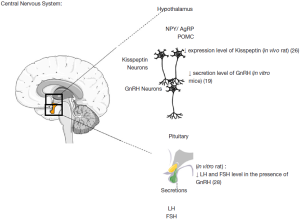
Fertility is controlled centrally at least in part by gonadotropin-releasing hormone (GnRH) neurones that are located in the hypothalamus. These neurons secrete GnRH which stimulate luteinizing hormone (LH) and follicle stimulating hormone (FSH) secretions by the pituitary. GnRH neurones are responsive to numerous stimuli including peripheral metabolic signals such as glucose and leptin (17,18). AMPK has been widely studied in the brain as it is involved in regulating food intake, a function regulated by the hypothalamus (19-22). The various anorectic signals (leptin, insulin, glucose) reduce AMPK activity whereas the orexigenic signals (ghrelin, neuropeptide Y) increase AMPK activity which permits regulation of food intake (23,24). In the brain, metformin inhibits AMPK activity in the hypothalamus and neuropeptide Y neurons which explains the appetite supressing nature of AMPK (25). In the same region of the brain, the functioning of neurons to kisspeptin and to GnRH seems also to be AMPK dependent. Intracerebroventricular (ICV) injection of another AMPK activator, AICAR, inhibits 60% of the kisspeptin-positive neurons from the arcuate nucleus of the hypothalamus, and conversely injection of an AMPK inhibitor, compound C, increases 2-fold the number of kisspeptin positive neurons (26). The ICV injection of AICAR in rat stimulated hypothalamic AMPK activation and increased the duration of the period of oestrus and reduced the inter-estrus interval (19). In ewes, injection of two activators of AMPK (metformin and AICAR) reduced the amplitude of the circadian rhythm of melatonin which is a key regulator in the control seasonality of reproduction (27). As a consequence, AMPK is a key participant in the operation of important reproductive neuron regulators (Figure 1).
AMPK has previously been shown to be present in the rat pituitary (28). While there appears to be no effect on FSH secretion (29), in women administered metformin for treatment with PCOS a reduction in LH secretion is observed (29-31). This reduction appears to be due to a reduction in pulse amplitude and not pulse frequency (31) and it is accepted that menstrual cycles can be corrected with metformin treatment (32,33), which indicates restoration of the hypothalamic—pituitary axis. This is in contrast to the rat where Tosca et al. (28) observed a reduction in both FSH and LH, and Lu et al. (34) observed a reduction in LH secretion. When activated by metformin, AMPK inhibited GnRH-stimulated FSH and LH release by a MAPK3/1 mediated pathway and activin-stimulated FSH release by a SMAD2 dependent pathway (28). These reductions in gonadotropin secretion would likely then result in diminished steroid synthesis in the ovary.
Metformin and the adult testis
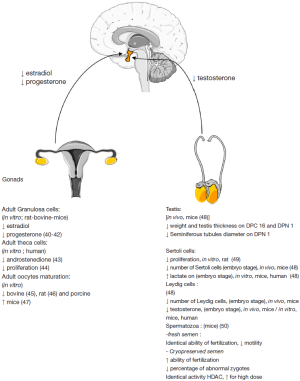
Male fertility is influenced by nutrition and energy metabolism. Indeed, we find among marathoners who have little energy reserve (35) or conversely in cases of obesity, reduced sperm production (36). As early as the 1970’s, the relationship between lipid metabolism and male fertility was addressed through the use of linoleic acid (37). More recently with the use of animal models, it has been possible to show that the synthesis of polyunsaturated fatty acids (PUFA) with long-chains are present in cell membranes (including sperm), and are provided by the transformation of essential PUFA n-3 and n-6 by the Sertoli cells in rats (38).
There is evidence that metformin inhibits complex I of the mitochondria respiratory chain thereby reducing mitochondrial function and cellular respiration, leading to anaerobic respiration and an increase in lactate secretion (7). Lactate is the primary energy substrate for male germ cells and is produced by the Sertoli cells. Thus, stimulation of rat Sertoli cells with metformin or AICAR-mediated AMPK activation resulted in a 3-fold increase in lactate production (39). Together these results suggest a role for AMPK and metformin modulating the nutritional function of Sertoli cells (Figure 2). In accordance with this production, we found that mRNA level of glucose transporter Glut1 and lactate dehydrogenase LDH, which catalyzed the conversion of pyruvate to lactate, tended to increase. Similarly, upregulation of Glut1 and LDH expression has also been described in rat Sertoli cells after AICAR treatment (39).
Metformin administered to rabbits with alloxan-induced (type 1) diabetes has been demonstrated to reduce testicular weight, sperm cell count, sperm motility, and an increase in the number of dead and abnormal sperm (51), suggesting adverse effects on male fertility. Furthermore, the long-term use of some anti-diabetic drugs has been reported to produce mutagenicity and nuclear damage (52,53). In addition, the treatment of sperm with a known natural activator of AMPK, resveratrol reduced DNA damage and lipid peroxidation (54-56). As metformin is administered to males for diabetes, it was pertinent to assess the effects of metformin on sperm function and fertilizing capacity when it was added in sperm cryopreservation media of mouse sperm (50). Under the conditions of our study, the results demonstrated that the presence of metformin in the cryopreservation media induced an improvement in the fertilization rate and a reduction in the proportion of abnormal embryos after in vitro fertilization (50). This increase in AMPK activity was associated with an increase in IVF success that could be linked to results described in a species of arctic frog (Rana sylvatica) with high cryotolerance (57). Interestingly, during the freezing of frog tissues, AMPK activity increases 2.5- to 4.4- fold (57,58), suggesting that AMPK is involved in the natural responses to freezing and thawing. In contrast, the results obtained with fresh sperm in Bertoldo et al. are in accordance to those of Hurtado de Llera et al. (59) who observed a partial reduction in motility of boar sperm following 5,000 µM treatment with metformin in fresh sperm for two hours.
Maternal insulin resistance and management of Reproduction and Pregnancy
Metformin and the adult ovary—PCOS
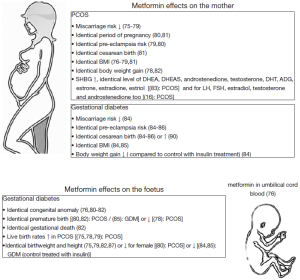
PCOS affects 5% to 10% of women in reproductive age and represents the most common cause of infertility (75% of cases of anovulatory infertility) that is associated with anovulation, hyperandrogenism and metabolic disturbance (60). Considerable research effort has been directed towards the administration of metformin for the treatment of PCOS (61). Furthermore, the therapeutic strategy facing a patient presenting with PCOS is in general, well defined.
Clomiphene citrate, an estrogen receptor inhibitor, is used to restore ovulation and thus promote follicle development in these patients by reducing the negative feedback effects of estrogen on the hypothalamus. Then metformin usually prescribed in the treatment of type 2 diabetes, may be administered for its insulin-sensitizing properties, and to facilitate the decrease circulating levels of androgens (43). In this context, metformin is given to improve the effectiveness of clomiphene citrate (62). To clarify the mechanism of metformin’s action on the cell types implicated in ovarian function, several research groups have studied the activity of AMPK in the different ovarian cell types. AMPK is present in all cell compartments of the ovary in all the domestic species studied to date (cow, goat, sheep and pig), the rat, humans and also in chickens. Expression could be changed in function the maturation stage. In hens, AMPK phosphorylation decreases in granulosa cells during terminal folliculogenesis, suggesting a role of this kinase during the pre-ovulatory period (Figure 2) (40).
Different studies have been conducted to determine if the action of metformin on the ovary is direct or indirect (28,40,41,63). Even if the mechanism of metformin action is still unclear, several studies have suggested actions of metformin in granulosa cell steroidogenesis and oocyte maturation (45). In adolescent girls with precocious onset of puberty, metformin administration has permitted a reduction in excess androgens (64), several studies have shown that metformin can lead to a diminution of systemic androgen in women experiencing PCOS (65,66). However, others authors have observed that results are not due to metformin alone (67).
Similar observations mainly in vivo or in ovarian cell culture models have been described in several species (human, rodents, cow and goat) where reductions of estradiol, progesterone from the granulosa and even androgen by human thecal cells was observed (42,43) in vivo and in vitro (68). The reduction in progesterone synthesis in the presence of metformin only occurs following the stimulation of cells with either FSH, IGF-1 alone, or together (41,42). Metformin exerts an effect on the gene expression of proteins involved in steroid production (CYP11A1, 3βHSD, aromatase) as we have reported by qRT-PCR analysis, but also at the protein activity level in bovine granulosa cells (41,42). In addition, in rat primary granulosa cells, metformin reduced estradiol and progesterone after only 3 h of stimulation (41). Metformin is also capable of stimulating in vitro lactate production by human granulosa cells (69).
Following the incubation of a human theca cell line with metformin, a reduction in the synthesis of androstenedione was observed (43). In cultures of primary cells of theca interna from the rat, the addition of metformin was correlated with increased activity of AMPK and inhibition of proliferation induced by insulin stimulation (44). However, in the latter study, the authors did not assess steroidogenesis. Metformin may therefore lead to decreased androgen synthesis through activation of AMPK and a reduction in proliferation of theca cells. Consequently, less aromatizable androgen would be available to granulosa cells to produce estradiol. Indeed in the rat, the activation of AMPK in granulosa cells inhibits their proliferation (46,70). As observed in granulosa cells, AMPK is involved in the luteal synthesis of progesterone. In the bovine corpus luteum, LH activity inhibits AMPK activation as the AMPK activator AICAR decreases the synthesis of LH-stimulated progesterone in the cow (71).
In addition, adipokines which are cytokines produced mainly by adipose tissue and also by the gonads could modify steroid production. Thus, it was recently demonstrated that visfatin is present in all cellular compartments of the ovary and that metformin increases visfatin expression via AMPK and SIRT1 in human granulosa cells (72). In addition, visfatin increased IGF1- but not FSH-induced steroid secretion (72), highlighting another level of regulation on ovarian steroidogenesis by metformin and AMPK.
Another action of metformin is its involvement in oocyte maturation. Indeed, in bovine and porcine oocytes, metformin blocked meiotic progression to the germinal vesicle stage (45,73). In bovine oocytes, meiotic arrest was associated with increased AMPK activity, reduced MAPK3/1 phosphorylation in both oocytes and cumulus cells, and the latency of two key factors (RPS6 and EEF2) involved in protein synthesis in oocytes. In addition, these effects were only evident in cumulus-oocyte complexes and not in denuded oocytes, indicating that at least in the bovine, cumulus cells are essential for metformin to exert an effect on the oocyte (45). Furthermore in the marine worm, Cerebratulus lacteus, as in the bovine and porcine models, oocyte maturation is correlated with the inhibition of the AMPK complex (74). As mouse oocytes do not require rounds of protein synthesis, species effect differences may be attributed to an inhibitory effect on key proteins for meiotic progression. More studies will be needed to determine the role of AMPK as it appears there are significant differences between species.
Metformin and gestational diabetes
In contrast to metformin administration for PCOS (Figure 3), metformin in women with gestational diabetes is absent around the time of terminal oocyte maturation, conception and early embryo development with in utero exposure occurring in the second and third trimesters. The placenta plays a central role in the transfer of drugs between the mother and the unborn young and metformin could directly affect fetal physiology and embryonic development as it crosses the placenta (89,90). Despite this, metformin is currently listed as a category B drug for use during pregnancy, meaning that animal reproduction studies (91) have failed to demonstrate a risk to the fetus, yet there are no adequate and well-controlled studies in pregnant women (92). Thus, metformin has been detected in umbilical cord blood at levels equal to that in maternal venous blood (93). Hughes et al. observed that clearance of metformin in women with type 2 diabetes increases during pregnancy as a result of enhanced renal clearance (94). Consequently, although there appears to be a paucity of information available, the results suggest that to maintain a therapeutic effect, a greater than 20% increase in the dose is perhaps required (92,94), resulting in greater concentrations of systemic metformin and increased fetal exposure (95).
Recent data have observed that use of insulin sensitizers and metformin, during the first 12 weeks of gestation or more, reduced development of gestational diabetes and did not influence the health of babies, and no obstetric complications or congenital anomalies were described (Figure 3) (80). Appropriate metformin therapy during gestational diabetes can decrease both maternal and fetal morbidity, particularly congenital abnormalities, neonatal hypoglycemia and macrosomia, and fetal loss (85,96,97). Data suggest that metformin used during the first gestational trimester is not teratogenic (75,82,98,99). However, metformin has been reported to increase the rate of preterm birth (100). Infants of diabetic mothers exposed to metformin in utero can experience a reduction in insulin resistance, which may have an advantageous effect on adipose tissue distribution. When mothers with gestational diabetes were administered either metformin or insulin, metformin exposed children had larger measures of subcutaneous fat, but overall body fat was the same two years after the birth of the children (101). However, no differences were observed between the two groups in terms of central fat measures, total fat mass or percentage of body fat. Compared to those children exposed to insulin in utero, those exposed to metformin had larger upper arm circumference and bigger biceps and subscapular skin folds, indicating a more favorable pattern of fat distribution, and consequently less predisposed to insulin resistance and the adverse metabolic consequences of obesity than children exposed to insulin (92,101). These results have been supported by smaller but comparable studies (84-87,100,102). Overall metformin did not increase the risk of neonatal complications and may have been responsible for a decrease in neonatal hypoglycemia compared with insulin treatment.
With most findings consistent between studies, the use of metformin during gestational diabetes appears favorable. While there were inconsistencies in the rate of preterm births (100), all the above studies confirm that metformin is safe, with no significant maternal or neonatal adverse effects, and that metformin has improved neonatal outcomes compared with insulin. However, critically these studies lack the proper controls in terms of administration of neither metformin nor insulin which are needed to assess the long-term health of offspring. In spite of the overall available evidence, the literature is still inadequate to establish the long-term safety on offspring.
Metformin and fetal development
Metformin was in some studies administrated at the beginning of pregnancy, because, metformin treatment during pregnancy reduced the occurrence of miscarriage (75-79). However over an extended period, studies have observed that metformin has the ability to cross the placenta (89,90), and its use during pregnancy raises concerns about potential adverse effects on both the mother and fetus (91,92,103). Some studies have observed that in utero exposure to metformin early in pregnancy does not appear to cause any adverse effects or congenital malformations (75,78,79,81,82,99). Despite this, consequences on gonad development of the fetus have not been clearly studied.
Indeed, the first morphological sign of testicular differentiation is the maturation of Sertoli cells leading to the formation of testicular cords, 11.5 days post-coitum (dpc) in mouse and between 6-7 weeks post-fertilization in human. Subsequently, fetal leydig cells secrete testosterone from 12 dpc in mice (104-106). Within the seminiferous cords, germ cells called gonocytes proliferate and give rise to spermatogonial stem cells during postnatal life. The integrity of gonocytes is crucial for fertility in adulthood and for avoiding transmission of genetic modifications. Thus, when altered fetal androgen production interferes with masculinization it can result in disorders of sexual differentiation, phenotypic sex reversal, or more commonly, cryptorchidism and hypospadias. These disorders are recognized to be risk factors for low sperm counts and development of testicular cancer in adulthood (107).
At birth, babies from mothers treated by metformin to reduce the risk of gestational diabetes or treatment of fertility in case of PCOS do not present malformation or locomotor abnormalities (75,78,79,81,82,99). Even at three, six and eighteen months of life, metformin did not cause any adverse effects in terms of weight and motor skills (Figure 3) (80). Conversely at twelve months of age, Carlsen et al. (88) observed in utero metformin-exposed children were heavier when compared to placebo treated children. In a longitudinal study of the offspring of mothers treated with metformin for PCOS, there were no differences in height, weight or body composition at eight years of age between those exposed to metformin or the placebo (108). In addition, after controlling for pregnancy complications, neonatal hypoglycemia (82) and the mean birthweight percentile of neonates exposed to metformin (81) was significantly lower than that of neonates delivered to normal healthy matched controls. Evidently, further research is required to confirm or disprove these and other potential effects of in utero exposure of metformin later in life (108).
During fetal testis development, two main functions take place: gametogenesis and steroidogenesis. The commencement of testosterone production is important as it is involved in masculinization. The first trimester of pregnancy is a window where gonads are sensitive to exogenous substances due to the first step of gonad differentiation. Preliminary human evidence has shown, that compared to non-exposed controls, metformin exposed offspring have not been reported to differ in male and female steroids (83) or anti-müllerian hormone (AMH), a hormone involved in the differentiation of the male tract (109). In spite of these reports, the free testosterone index (FTI), which is the ratio used to evaluate the androgen status in humans and is calculated by the total testosterone level divided by the sex hormone binding globulin (SHBG) level, was increased in metformin-exposed male offspring (109). In addition, an increase in levels in SHBG newborns exposed to metformin during the first trimester of pregnancy has also been reported (83). It would be anticipated that in an increase in SHBG would result in a lower free androgen indexes (FAI). SHBG is a glycoprotein that binds androgens and estradiol and modulates their biological properties. This premise supports the results of the Carlsen and Vanky study (83) but does not explain those of Vanky and Carlsen (109) which remain unsettled. However, in vitro studies and in vivo experiments in mouse models have reported on the consequences on androgens production. Organotypic culture systems using human and mouse testis demonstrated similar effects of metformin where a reduction in testosterone synthesis but an increase in lactate production was observed (48). Lactate is an energy substrate for germ cells. Nonetheless, the human testis is more sensitive to metformin than mouse testis. Hence, a concentration of only 50 µM metformin in human testis culture leads to a 45% decrease in testosterone secretion whereas 500 µM of metformin is needed in mouse culture to obtain a 20% decrease in testosterone release (48). So even if testosterone synthesis is reduced and another marker called, Insl3, mRNA is greatly inhibited by metformin in mouse testicular explants in vitro, no alteration of testis descent was observed in these mice treated in utero (48). Given the results, it could be interesting to measure the anogenital distance in new born males which is considered a marker of fetal androgen action (48).
An important consequence associated with these modifications of the endocrine system was that in utero metformin-exposed new borns presented with a decrease in testis size, tubule diameter and a lower Sertoli cells number than control mice (48). Certainly, the Sertoli cell population determines the efficiency of spermatogenesis (110), and there is a strong correlation between the number of Sertoli cells, the number of germ cells interacting with them, sperm production and finally the testicular volume. These results raise some questions about harmlessness of metformin on gonad development and the fertility of the progeny from treated mothers, where we have no data at this time.
Metformin & epigenetics
In mice, oocyte maturation in vitro could be stimulated by activation of AMPK. AMPK is involved in oocyte for condensation of chromatin and formation of the meiotic spindle at the resumption of meiosis (47,111). Thus, AMPK has been shown to directly associate with chromatin to regulate transcriptional programs required to respond and survive to a wide variety of environmental stresses (112). It exerts its effect on the chromatin by phosphorylating histone H2B at serine 36 or by regulating histone deacetylases (HDAC), a class of enzymes that remove acetyl groups on histones. When this process is perturbed, lower cell survival and response to stress is observed (112). We have shown that genetic ablation of AMPKα1 in Sertoli cells results in reduced phosphorylated histone H2B associated with a reduction in Sertoli cell function and reduced sperm function and fertility. Together these results highlight the importance of AMPK in responding to stress and proper cell function and the potential long-term effects if AMPK signaling is perturbed.
While the immediate effects of metformin in the treatment of PCOS and diabetes have been relatively well characterised, data on the longer term effects of metformin is lacking. Salomäki et al. (91) hypothesized that prenatal metformin exposure possibly has longer term effects on the offspring. In mice it was demonstrated that fetal metformin exposure programs the metabolic phenotype of the offspring. This included an increase in fetal weight at day 18 post-coitum without affecting maternal weight development or food intake. When the progeny were fed a high fat diet, prenatal metformin exposure led to increased body weight gain, a reduction in total body water content and an increase in mesenteric fat and liver weight at the end of the high fat diet phase (91). In addition, male offspring exposed to metformin exhibited impaired glucose tolerance and elevated fasting glucose during the high fat diet.
Caton et al. (113) proposed that metformin increased hepatic SIRT1 activity, an histone deacetylase, through AMPK-mediated induction of nicotinamide phosphoribosyltransferase (NAMPT). Recently we assessed the effects of metformin supplementation in the cryopreservation media of mouse sperm (50). The activity of SIRT1, which can be modulated by metformin through AMPK, was increased in the presence of pharmacological doses of metformin. HDACs are present in elongated spermatids and spermatozoa at various stages of development (114-116). It has previously been reported that HDAC deacetylates some proteins associated with mitochondrial function or metabolism such as CRTC2 or PGC1α, but also histones. It will be necessary to determine the exact role of metformin in the proper programming of the epigenome as our results suggest that metformin has the capability of altering histone acetylation (50).
Conclusions
The literature examined in the present review, support the premise that metformin is safe to use during pregnancy with respect to immediate pregnancy outcomes. However, while the preliminary results of longitudinal studies in progress are encouraging, we still do not have adequate data to say with any certainty that metformin does not have any adverse effects for whole of life health. Indeed, when reviewing the literature it becomes apparent that numerous methodological issues are relevant since they affect the interpretation of some of the studies published on pregnancy outcomes (92,117,118). Presently most studies have been conducted retrospectively and conducted at various centers across many countries, in addition many studies include a small sample size, thus limiting the validity of results. Nonetheless, advancements in our understanding in the molecular modes of action of metformin are progressing. Whether pregnant women should be administered metformin remains controversial (92). While there is no evidence of teratogenicity associated with metformin treatment when used during pregnancy, data presented in the present review raise questions of safety in terms of fetal testicular development. Ambiguity in the results of studies will ensure that this controversy remains.
Acknowledgements
Melanie Faure was supported by the Region Centre and Institut National de la Recherche Agronomique. This work was financially supported by Institut National de la Recherche Agronomique (INRA) and the national program FERTiNERGY funded by the French National Research Agency (ANR).
Disclosure: The authors declare no conflict of interest.
References
- Simmons D. Metformin treatment for Type 2 diabetes in pregnancy? Best Pract Res Clin Endocrinol Metab 2010;24:625-34. [PubMed]
- Hawthorne G. Metformin use and diabetic pregnancy-has its time come? Diabet Med 2006;23:223-7. [PubMed]
- Wilcock C, Wyre ND, Bailey CJ. Subcellular distribution of metformin in rat liver. J Pharm Pharmacol 1991;43:442-4. [PubMed]
- Wilcock C, Bailey CJ. Accumulation of metformin by tissues of the normal and diabetic mouse. Xenobiotica 1994;24:49-57. [PubMed]
- Detaille D, Guigas B, Levere X, et al. Obligatory role of membrane events in the regulatory effect of metformin on the respiratory chain function. Biochem Pharmacol 2002;63:1259-72. [PubMed]
- Shu Y, Sheardown SA, Brown C, et al. Effect of genetic variation in the organic cation transporter 1 (OCT1) on metformin action. J Clin Invest 2007;117:1422-31. [PubMed]
- Owen MR, Doran E, Halestrap AP. Evidence that metformin exerts its anti-diabetic effects through inhibition of complex 1 of the mitochondrial respiratory chain. Biochem J 2000;348:607-14. [PubMed]
- Zhou G, Myers R, Li Y, et al. Role of AMP-activated protein kinase in mechanism of metformin action. J Clin Invest 2001;108:1167-74. [PubMed]
- Viollet B, Guigas B, Leclerc J, et al. AMP-activated protein kinase in the regulation of hepatic energy metabolism: from physiology to therapeutic perspectives. Acta Physiol (Oxf) 2009;196:81-98. [PubMed]
- Hardie DG, MacKintosh RW. AMP-activated protein kinase--an archetypal protein kinase cascade? Bioessays 1992;14:699-704. [PubMed]
- Rattan R, Giri S, Hartmann LC, et al. Metformin attenuates ovarian cancer cell growth in an AMP-kinase dispensable manner. J Cell Mol Med 2011;15:166-78. [PubMed]
- Foretz M, Hebrard S, Leclerc J, et al. Metformin inhibits hepatic gluconeogenesis in mice independently of the LKB1/AMPK pathway via a decrease in hepatic energy state. J Clin Invest 2010;120:2355-69. [PubMed]
- Kalender A, Selvaraj A, Kim SY, et al. Metformin, independent of AMPK, inhibits mTORC1 in a rag GTPase-dependent manner. Cell Metab 2010;11:390-401. [PubMed]
- Nestler JE, Jakubowicz DJ, Evans WS, et al. Effects of metformin on spontaneous and clomiphene-induced ovulation in the polycystic ovary syndrome. N Engl J Med 1998;338:1876-80. [PubMed]
- Vandermolen DT, Ratts VS, Evans WS, et al. Metformin increases the ovulatory rate and pregnancy rate from clomiphene citrate in patients with polycystic ovary syndrome who are resistant to clomiphene citrate alone. Fertil Steril 2001;75:310-5. [PubMed]
- De Leo V, la Marca A, Ditto A, et al. Effects of metformin on gonadotropin-induced ovulation in women with poly-cystic ovary syndrome. Fertil Steril 1999;72:282-5. [PubMed]
- Pal L, Chu HP, Shu J, et al. In vitro evidence of glucose-induced toxicity in GnRH secreting neurons: High glucose concentrations influence GnRH. Fertil Steril 2007;88:1143-9. [PubMed]
- Gamba M, Pralong FP. Control of GnRH neuronal activity by metabolic factors: the role of leptin and insulin. Mol Cell Endocrinol 2006;254-255:133-9. [PubMed]
- Coyral-Castel S, Tosca L, Ferreira G, et al. The effect of AMP-activated kinase activation on gonadotrophin-releasing hormone secretion in GT1-7 cell and its potential role in hypothalamic regulation of the oestrous cyclicity in rats. J Neuroendocrinol 2008;20:335-46. [PubMed]
- Andersson U, Flilipsson K, Abbott CR, et al. AMP-activated protein kinase plays a role in the control of food intake. J Biol Chem 2004;279:12005-8. [PubMed]
- Han SM, Namkoong C, Jang PG, et al. Hypothalamic AMP-activated protein kinase mediates counter-regulatory responses to hypoglycaemia in rats. Diabetologia 2005;48:2170-8. [PubMed]
- Kim EK, Miller I, Aja S, et al. C75, a fatty acid synthase inhibitor, reduces food intake via hypothalamic AMP-activated protein kinase. J Biol Chem 2004;279:19970-6. [PubMed]
- Claret M, Smith MA, Batterham RL, et al. AMPK is essential for energy homeostasis regulation and glucose sensing by POMC and AgRP neurons. J Clin Invest 2007;117:2325-36. [PubMed]
- Minokoshi Y, Alquier T, Furukawa N, et al. AMP-kinase regulates food intake by responding to hormonal and nutrient signals in the hypothalamus. Nature 2004;428:569-74. [PubMed]
- Chau-Van C, Gamba M, Salvi R, et al. Merformin inhibits adenosine 5'-monophosphate-activated kinase activation and prevents increases in neuropeptide Y expression in cultured hypothalamic neurons. Endocrinology 2007;148:507-11. [PubMed]
- Wen JP, Liu C, Bi WK, et al. Adiponectin inhibits KISS1 gene transcription through AMPK and specificity protein-1 in the hypothalamic GT1-7 neurons. J Endocrinol 2012;214:177-89. [PubMed]
- Menassol JB, Tautou C, Collet A, et al. The effect of an intracerebroventricular injection of metformin or AICAR on the plasma concentrations of melatonin in the ewe: potential involvement of AMPK? BMC Neurosci 2011;12:76. [PubMed]
- Tosca L, Froment P, Rame C, et al. Metformin decreases GnRH- and activin-induced gonadotropin secretion in rat pituitary cells: potential involvement of adenosine 5' monophosphate-activated protein kinase (PRKA). Biol Reprod 2011;84:351-62. [PubMed]
- Oride A, Kanasaki H, Purwana IN, et al. Effects of metformin administration on plasma gonadotropin levels in women with infertility, with an in vitro study of the direct effects on the pituitary gonadotrophs. Pituitary 2010;13:236-41. [PubMed]
- Billa E, Kapolla N, Nicopoulou SC, et al. Metformin administration was associated with a modification of LH, prolacting and insulin secretion dynanmics in women with polycystic ovarian syndrome. Gynecol Endocrinol 2009;25:427-34. [PubMed]
- Genazzani AD, Battaglia C, Malavasi B, et al. Metformin administration modulates and restores luteinizing hormone spontaneous episodic secretion and ovarian function in nonobese patients with polycystic ovary syndrome. Fertil Steril 2004;81:114-9. [PubMed]
- Glueck CJ, Wang P, Fontaine R, et al. Metformin to restore normal menses in oligo-amenorrheic teenage girls with polycystic ovary syndrome (PCOS). J Adolesc Health 2001;29:160-9. [PubMed]
- Glueck CJ, Wang P, Fontaine R, et al. Metformin-induced resumption of normal menses in 39 of 43 (91%) previously amenorrheic women with the polycystic ovary syndrome. Metabolism 1999;48:511-9. [PubMed]
- Lu M, Tang Q, Olefsky JM, et al. Adiponectinb activates adenosine monophosphate-activated kinase and decreases luteinizing hormone secretion in LbetaT2 gonadotropes. Mol Endocrinol 2008;22:760-71. [PubMed]
- De Souza MJ, Arce JC, Pescatello LS, et al. Gonadal hormones and semen quality in male runners. A volume threshold effect of endurance training. Int J Sports Med 1994;15:383-91. [PubMed]
- Hammoud AO, Wilde N, Gibson M, et al. Male obesity and alteration in sperm parameters. Fertil Steril 2008;90:2222-5. [PubMed]
- Collier JG, Flower RJ, Stanton SL. Seminal prostaglandins in infertile men. Fertil Steril 1975;26:868-71. [PubMed]
- Retterstøl K, Haugen TB, Tran TN, et al. Studies on the metabolism of essential fatty acids in isolated human testicular cells. Reproduction 2001;121:881-7. [PubMed]
- Galardo MN, Riera MF, Pellizzari EH, et al. The AMP-activated protein kinase activator, 5-aminoimidazole-4-carboxamide-1-b-D-ribonucleoside, regulates lactate production in rat Sertoli cells. J Mol Endocrinol 2007;39:279-88. [PubMed]
- Tosca L, Crochet S, Ferré P, et al. AMP-activated protein kinase activation moduclates progesteone secretion in granulosa cells from hen preovulatory follicles. J Endocrinol 2006;190:85-97. [PubMed]
- Tosca L, Solnais P, Ferre P, et al. Metformin-induced stimulation of adenosine 5' monophosphate-activated protein kinase (PRKA) impairs progesterone secretion in rat granulosa cells. Biol Reprod 2006;75:342-51. [PubMed]
- Tosca L, Chabrolle C, Uzbekova S, et al. Effects of metformin on bovine granulosa cells steroidogenesis: possible involvement of adenosine 5' monophosphate-activated protein kinase (AMPK). Biol Reprod 2007;76:368-78. [PubMed]
- Attia GR, Rainey WE, Carr BR. Metformin directly inhibits androgen production in human thecal cells. Fertil Steril 2001;76:517-24. [PubMed]
- Will MA, Palaniappan M, Peegel H, et al. Metformin: Direct inhibition of rat ovarian theca-interstitial cell proliferation. Fertil Steril 2012;98:207-14. [PubMed]
- Tosca L, Uzbekova S, Chabrolle C, et al. Possible role of 5' AMP-activated protein kinase in the metformin-mediated arrest of bovine oocytes at the germinal vesicle stage during in vitro maturation. Biol Reprod 2007;77:452-65. [PubMed]
- Kayampilly PP, Menon KMJ. AMPK activation by dihydrotestosterone reduces FSH-stimulated cell proliferation in rat granulosa cells by inhibiting ERK signaling pathway. Endocrinology 2012;153:2831-8. [PubMed]
- Downs SM, Davis CC. Role of AMPK throughout meiotic maturation in the mouse oocyte: Evidence for promoation of polar body formation and supression of premature activation. Mol Reprod Dev 2010;77:888-99. [PubMed]
- Tartarin P, Moison D, Guibert E, et al. Metformin exposure affects human and mouse fetal testicular cells. Hum Reprod 2012;27:3304-14. [PubMed]
- Riera MF, Regueira M, Galardo MN, et al. Signal transduction pathways in FSH regulation of rat Sertoli cell proliferation. Am J Physiol Endocrinol Metab 2012;302:E914-23. [PubMed]
- Bertoldo MJ, Guibert E, Tartarin P, et al. Effect of metformin on the fertilizing ability of mouse spermatozoa. Cryobiology 2014;68:262-8. [PubMed]
- Naglaa ZHE, Hesham AM, Fadil HA, et al. Impact of metformin on immunity and male fertility in rabbits with alloxan-induced diabetes. Journal of American Science 2010;6:417-26.
- Renner HW, Münzner R. Mutagenicity of sulphonylureas. Mutat Res 1980;77:349-55. [PubMed]
- Bedir A, Aliyazicioglu Y, Kahraman H, et al. Genotoxicity in rats treated with the antidiabetic agent, rosiglitazone. Environ Mol Mutagen 2006;47:718-24. [PubMed]
- Branco CS, Garcez ME, Pasqualotto FF, et al. Resveratrol and ascorbic acid prevent DNA damage induced by cryopreservation in human semen. Cryobiology 2010;60:235-7. [PubMed]
- Collodel G, Federico MG, Geminiani M, et al. Effect of trans-resveratrol on induced oxidative stress in human sperm and in rat germinal cells. Reprod Toxicol 2011;31:239-46. [PubMed]
- Garcez ME, dos Santos Branco C, Lara LV, et al. Effects of resveratrol supplementation on cryopreservation medium of human semen. Fertil Steril 2010;94:2118-21. [PubMed]
- Rider MH, Hussain N, Horman S, et al. Stress-induced activation of the AMP-activated protein kinase in the freeze tolerant frog Rana sylvatica. Cryobiology 2006;53:297-309. [PubMed]
- Storey KB. Reptile freeze tolerance: Metabolism and gene expression. Cryobiology 2006;52:1-16. [PubMed]
- Hurtado de Llera A, martin-Hidago D, Gil MC, et al. The AMPK activator metformin inhibits one of the main functions of boar spermatozoa, motility. FEBS Journal 2012;279 Suppl 1:52-576
- Dunaif A. Insulin resistance and the polycystic ovary syndrome: mechanism and implications for pathogenesis. Endocr Rev 1997;18:774-800. [PubMed]
- Misso ML, Costello MF, Garrubba M, et al. Metformin versus clomiphene citrate for infertility in non-obese women with polycyctic ovary syndrome: a systematic review and meta-analysis. Hum Reprod Update 2013;19:2-11. [PubMed]
- Kashyap S, Wells GA, Rosenwaks Z. Insulin-sensitizing agents as primary therapy for patients with polycystic ovarian syndrome. Hum Reprod 2004;19:2474-83. [PubMed]
- Rice S, Pellatt L, Ramanathan K, et al. Metformin inhibits aromatase via an extracellular signal-regulated kinase-mediated pathway. Endocrinology 2009;150:4794-801. [PubMed]
- Ibáñez L, López-Bermejo A, Díaz M, et al. Early metformin therapy (age 8-12 years) in girls with precocious pubarche to reduce hirsutism, androgen excess, and oligomenorrhea in adolescence. J Clin Endocrinol Metab 2011;96:E1262-7. [PubMed]
- Palomba S, Falbo A, Russo T, et al. Systemic and local effects of metformin administration in patients with polycystic ovary syndrome (PCOS): relationship to the ovulatory response. Hum Reprod 2010;25:1005-13. [PubMed]
- Kocak I, Ustün C. Effects of metformin on insulin resistance, androgen concentration, ovulation and pregnancy rates in women with polycystic ovary syndrome following laparoscopic ovarian drilling. J Obstet Gynaecol Res 2006;32:292-8. [PubMed]
- Bouchard P. Treatment of infertility in women with polycystic ovary syndrome. Ann Endocrinol (Paris) 2010;71:225-7. [PubMed]
- Mansfield R, Galea R, Brincat M, et al. Metformin has direct effect on human ovarian steroidogenesis. Fertil Steril 2003;79:956-62. [PubMed]
- Richardson MC, Ingamells S, Simonis CD, et al. Stimulation of lactate production in human granulosa cells by metformin and potential involvement of adenosine 5' monophosphate-activated protein kinase. J Clin Endocrinol Metab 2009;94:670-7. [PubMed]
- Kayampilly PP, Menon KM. Follicle-stimulating hormone inhibits adenosine 5'-monophosphate-activated protein kinase activation and promotes cell proliferation of primary granulosa cells in culture through an Akt-dependent pathway. Endocrinology 2009;150:929-35. [PubMed]
- Hou X, Arvisais EW, Davis JS. Luteinizing hormone stimulates mammalian target of rapamycin signaling in bovine luteal cells via pathways independent of AKT and mitogen-activated protein kinase: modulation of glycogen synthase kinase 3 and AMP-activated protein kinase. Endocrinology 2010;151:2846-57. [PubMed]
- Reverchon M, Cornuau M, Cloix L, et al. Visfatin is expressed in human granulosa cells: regulation by metformin through AMPK/SIRT1 pathways and its role in steroidogenesis. Mol Hum Reprod 2013;19:313-26. [PubMed]
- Mayes MA, Laforest MF, Guillemette C, et al. Adenosine 5'-monophosphate kinase-activated protein kinase (PRKA) activators delay meiotic resumption in porcine oocytes. Biol Reprod 2007;76:589-97. [PubMed]
- Stricker SA. Potential upstream regulators and downstream targets of AMP-activated kinase signaling during oocyte maturation in a marine worm. Reproduction 2011;142:29-39. [PubMed]
- Glueck CJ, Wang P, Goldenberg N, et al. Pregnancy outcomes among women with polycystic ovary syndrome treated with metformin. Hum Reprod 2002;17:2858-64. [PubMed]
- Jakubowicz DJ, Iuorno MJ, Jakubowicz S, et al. Effects of metformin on early pregnancy loss in the polucystic ovary syndrome. J Clin Endocrinol Metab 2002;87:524-9. [PubMed]
- Khattab S, Mohsen IA, Foutouh IA, et al. Metformin reduces abortion in pregnant women with polycystic ovary syndrome. Gynecol Endocrinol 2006;22:680-4. [PubMed]
- Nawaz FH, Khalid R, Naru T, et al. Does continuous use of metformin throughout pregnancy improve pregnancy outcomes in women with poycystic ovarian syndrome. J Obstet Gynaecol Res 2008;34:832-7. [PubMed]
- De Leo V, Musacchio MC, Piomboni P, et al. The administration of metformin during pregnancy reduces polycystic ovary syndrome related gestational complications. Eur J Obstet Gynecol Reprod Biol 2011;157:63-6. [PubMed]
- Glueck CJ, Goldenberg N, Pranikoff J, et al. Height, weight, and motor-social development during the first 18 months of life in 126 infants born to 109 mothers with polycystic ovary syndrome who conceived on and continued metformin through pregnancy. Hum Reprod 2004;19:1323-30. [PubMed]
- Kovo M, Weissman A, Gur D, et al. Neonatal outcome in polycystic ovarian syndrome patients treated with metformin during pregnancy. J Matern Fetal Neonatal Med 2006;19:415-9. [PubMed]
- Bolton S, Cleary B, Walsh J, et al. Continuation of metformin in the first trimester of women with polycystic ovarian syndrome is not associated with increased perinatal morbidity. Eur J Pediatr 2009;168:203-6. [PubMed]
- Carlsen SM, Vanky E. Metformin influence on hormone levels at birth, in PCOS mothers and their newborns. Hum Reprod 2010;25:786-90. [PubMed]
- Niromanesh S, Alavi A, Sharbaf FR, et al. Metformin compared with insulin in the management of gestational diabetes mellitus: a randomized clinical trial. Diabetes Res Clin Pract 2012;98:422-9. [PubMed]
- Gandhi P, Bustani R, Madhuvrata P, et al. Introduction of metformin for gestational diabetes mellitus in clinical practice: Has it had an impact? Eur J Obstet Gynecol Reprod Biol 2012;160:147-50. [PubMed]
- Tertti K, Ekblad U, Vahlberg T, et al. Comparison of metformin and insulin in the treatment of gestational diabetes: a retrospective, case-control study. Rev Diabet Stud 2008;5:95-101. [PubMed]
- Ijäs H, Vääräsmaki M, Morin-Papunen L, et al. Metformin should be considered in the treatment of gestational diabetes: a prospective randomised study. BJOG 2011;118:880-5. [PubMed]
- Carlsen SM, Martinussen MP, Vanky E. Metformin’s effect on first-year weight gain: a follow-up study. Pediatrics 2012;130:e1222-6. [PubMed]
- Kovo M, Haroutiunian S, Feldman N, et al. Determination of metformin transfer across the human placenta using a dually perfused ex vivo placental cotyledon model. Eur J Obstet Gynecol Reprod Biol 2008;136:29-33. [PubMed]
- Vanky E, Zahlsen K, Spigset O, et al. Placental passage of metformin in women with polycystic ovary syndrome. Fertil Steril 2005;83:1575-8. [PubMed]
- Salomäki H, Vähätalo LH, Laurila K, et al. Prenatal metformin exposure in mice programs the metabolic phenotype of the offspring during a high fat diet at adulthood. PLoS One 2013;8:e56594. [PubMed]
- Lautatzis M-E, Goulis DG, Vrontakis M. Efficacy and safety of metformin during pregnancy in women with gestational diabetes mellitus or polycystic ovary syndrome: A systematic review. Metabolism 2013;62:1522-34. [PubMed]
- Charles B, Norris R, Xiao X, et al. Population pharmacokinetics of metformin in late pregnancy. Ther Drug Monit 2006;28:67-72. [PubMed]
- Hughes RC, Gardiner SJ, Begg EJ, et al. Effect of pregnancy on the pharmacokinetics of metformin. Diabet Med 2006;23:323-6. [PubMed]
- Eyal S, Easterling TR, Carr D, et al. Pharmacokinetics of metformin during pregnancy. Drug Metab Dispos 2010;38:833-40. [PubMed]
- Langer O, Yogev Y, Most O, et al. Gestational diabetes: the consequences of not treating. Am J Obstet Gynecol 2005;192:989-97. [PubMed]
- Crowther CA, Hiller JE, Moss JR, et al. Effect of treatment of gestational diabetes mellitus on pregnancy outcomes. N Engl J Med 2005;352:2477-86. [PubMed]
- Gutzin SJ, Kozer E, Magee LA, et al. The safety of oral hypoglycemic agents in the first trimester of pregnancy: a meta-analysis. Can J Clin Pharmacol 2003;10:179-83. [PubMed]
- Balani J, Hyer SL, Rodin DA, et al. Pregnancy outcomes in women with gestational diabetes with metformin or insulin: a case-control study. Diabet Med 2009;26:798-802. [PubMed]
- Rowan JA. Metformin versus insulin for the treatment of gestational diabetes. N Engl J Med 2008;358:2003-15. [PubMed]
- Rowan JA, Rush EC, Obolonkin V, et al. Metformin in gestational diaabetes: the offspring follow-up (MiG TOFU): body composition at 2 years. Diabetes Care 2011;34:2279-84. [PubMed]
- Ekpebegh CO, Coetzee EJ, van de Merwe L, et al. A 10-year retrospective analysis of pregnancy outcome in pregestational Type 2 diabetes: comparison of insulin and oral glucose-lowering agents. Diabet Med 2007;24:253-8. [PubMed]
- Harris EL. Adverse reactions to oral antidiabetic agents. BMJ 1971;3:29-30. [PubMed]
- Habert R, Lejeune H, Saez JM. Origin, differentiation and regulation of fetal and adult Leydig cells. Mol Cell Endocrinol 2001;179:47-74. [PubMed]
- Tapanainen J, Kellokumpu-Lehtinen P, Pelliniemi L, et al. Age-related changes in endogenous steroids of human fetal testis during early and midpregnancy. J Clin Endocrinol Metab 1981;52:98-102. [PubMed]
- Lambrot R, Livera G, Coffigny H, et al. A new method for toxicity assays on human and mouse fetal testis. Biochimie 2006;88:1831-5. [PubMed]
- Skakkebaek NE, Rajpert-De Meyts E, Main KM. Testicular dysgenesis syndrome: an increasingly common developmental disorder with environmental aspects. Hum Reprod 2001;16:972-8. [PubMed]
- Rø TB, Ludvigsen HV, Carlsen SM, et al. Growth, body composition and metabolic profile of 8-year-old children exposed to metformin in utero. Scand J Clin Lab Invest 2012;72:570-5. [PubMed]
- Vanky E, Carlsen SM. Androgens and antimullerian hormone in mothers with polycystic ovary syndrome and their newborns. Fertil Steril 2012;97:509-15. [PubMed]
- Petersen C, Soder O. The sertoli cell--a hormonal target and ’super’ nurse for germ cells that determines testicular size. Horm Res 2006;66:153-61. [PubMed]
- Chen J, Hudson E, Chi MM, et al. AMPK regulation of mouse oocyte meiotic resumption in vitro. Dev Biol 2006;291:227-38. [PubMed]
- Bungard D, Fuerth BJ, Zeng P-Y, et al. Signaling kinase AMPK activates stress-promoted transcription via histone H2B phosphorylation. Science 2010;329:1201-5. [PubMed]
- Caton PW, Nayumi NK, Kieswich J, et al. Metformin suppresses hepatic gluconeogenesis through induction of SIRT1 and GCN5. J Endocrinol 2010;205:97-106. [PubMed]
- Choi E, Han C, Park I, et al. A Novel Germ Cell-specific Protein, SHIP1, Forms a Complex with Chromatin Remodeling Activity during Spermatogenesis. J Biol Chem 2008;283:35283-94. [PubMed]
- Hazzouri M, Pivot-Pajot C, Faure A-K, et al. Regulated hyperacetylation of core histones during mouse spermatogenesis: involvement of histione-deacetylases. Eur J Cell Biol 2000;79:950-60. [PubMed]
- Yan W, Si Y, Slaymaker S, et al. Zmynd15 encodes a histone deacetylase-dependent transcriptional repressor essential for spermiogenesis and male fertility. J Biol Chem 2010;285:31418-26. [PubMed]
- Ghazeeri GS, Nassar AH, Younes Z, et al. Pregnancy outcomes and the effect of metformin treatment in women with polycystic ovary syndrome: an overview. Acta Obstet Gynecol Scand 2012;91:658-78. [PubMed]
- Norman RJ, Wang JX, Hague W. Should we continue or stop insulin sensitizing drugs during pregnancy? Curr Opin Obstet Gynecol 2004;16:245-50. [PubMed]