Minimally invasive transforaminal lumbar interbody fusion for lumbar spondylolisthesis
Case vignette
A 59-year-old woman with a history of persistent low back pain presents to the clinic with intermittent claudication and worsening right leg pain. The patient denies any bladder or bowel symptoms. On physical examination, there is bilateral lower extremity weakness specifically in the right extensor hallucis longus. The patient also demonstrates decreased sensation to light touch over the dorsum of the foot. Diagnostic magnetic resonance imaging (MRI) demonstrates an L5-S1 spondylolisthesis with spinal stenosis. Given the persistent neurological symptoms and evidence of spinal instability the patients is scheduled to undergo a minimally invasive transforaminal lumbar interbody fusion (MIS TLIF).
Surgical technique
With the patient prone on a standard Jackson table, the target level is identified via intraoperative fluoroscopy (Figure 1). The superior endplate of the vertebra cephalad to the target level (L5-S1) should be centered radiographically on an anterior-posterior (AP) view with the spinous process equidistant between the pedicles. A stab incision is then placed just lateral to the pedicle to be cannulated and a Jamshidi needle is advanced under AP fluoroscopy towards the intersection of the transverse process and superior facet. On the right side the start point corresponds to the 2 o’clock position, on the left side it is the 10 o’clock position. The lateral start point enables a medial trajectory thru the pedicle that preserves the facet joint.

Once the start position is confirmed, the Jamshidi needle is advanced in 5 mm increments thru the pedicle under AP fluoroscopic guidance. Caution is exercised to prevent a medial breach of the pedicle wall by the tip of the Jamshidi. When the Jamshidi has been advanced 20 mm the AP fluoroscopic image is checked to ensure the tip has not violated the medial pedicle wall. At 20 mm the Jamshidi is presumed to reach the posterior vertebral body wall. However, a lateral fluoroscopic image may be checked for confirmation. A guidewire is then advanced thru the Jamshidi into the vertebral body. If bilateral pedicle screws will be placed the contralateral side may be cannulated using a similar technique. The process is then repeated on the vertebra caudad to the target level.
On the side that the TLIF is to be performed, the superior and inferior stab incisions should be connected. The underlying muscular fascia is incised longitudinally and blunt sequential dilation is performed directed towards the lamina and pars of the disc space level. After achieving the desired working channel diameter, the cylindrical retractor should be secured to the table. The soft tissues overlying the bony elements are then resected with pituitaries and electrocautery to identify the interlaminar window, facet joint, and pars.
A laminectomy of the inferior aspect of the cephalad level is performed with a high-speed burr and extended cephalad until reaching the insertion of the ligamentum flavum. The bone can be saved in a bone trap to be used for the interbody fusion. The bony decompression can be extended medially by adjusting the tube to a more superficial depth and directed to the contralateral side. This will facilitate undercutting into the base of the spinous process and afford a view of the contralateral lateral recess. As the base of the spinous process is undercut a raphe in the ligamentum flavum will mark the midline. The bony decompression should extend across this raphe into the lateral recess of the opposite side to achieve full central and bilateral recess decompression.
The laminectomy should then be extended laterally to cut across the pars releasing the inferior facet of the cephalad level in a single large piece to expose the superior articular facet (SAF) (Figure 2). The SAF can then be resected in large pieces with the Kerrison punch along with the ligamentum flavum exposing the underlying dural sac and traversing nerve root. In some cases the caudal aspect of the exiting nerve root will become visible towards the lateral part of the facetectomy. If this occurs it should be carefully protected.
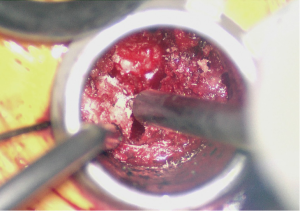
At this point the underlying disc space should be visible (Figure 3). The epidural veins are then coagulated with bipolar electrocautery and a 15 blade is utilized to perform an annulotomy. A Kerrison punch can help expand the annulotomy medially to assist in disc excision and end plate preparation. A discectomy is performed thru the annulotomy with a combination of disc shavers, pituitary rongeurs, and straight and curved curettes. Lateral fluoroscopy should be checked to ensure the anterior longitudinal ligament is not violated.
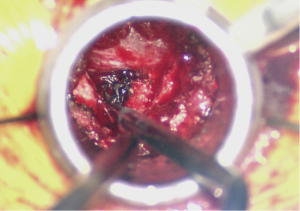
With the discectomy completed and the end plates thoroughly prepared, a trial cage can then be placed with the aim to restore lumbar lordosis. When the size is selected the interbody space can be packed with a combination of the locally collected autograft and bone graft extender or allograft. The interbody cage should also be packed with either autograft or another biologic and advanced into the disc space under fluoroscopic guidance (Figure 4). Great care should be taken to not cause any injury to the nerve roots. Depending on the type of cage, the tube may have to be loosened to accommodate for cage articulation.

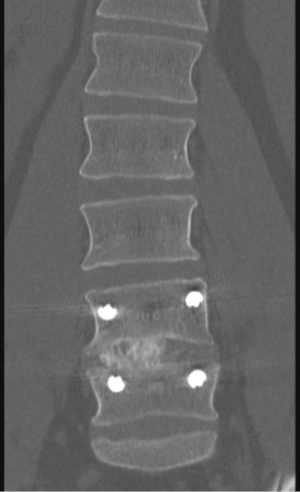
Posterior pedicle screw placement can then be performed percutaneously over the guidewires with the use of lateral fluoroscopic guidance. An appropriate length rod is then placed spanning the pedicle screws in a subfascial manner. AP and lateral fluoroscopy images should be checked after all instrumentation has been placed (Figures 5,6,7).


Comments
Minimally invasive spine surgery (MISS) has gained considerable momentum as growing evidence suggests comparable clinical outcomes to traditional open surgery with less postoperative morbidity, and faster patient recovery. Since the inception of MISS in the 1980s, significant advancements in instrumentation, imaging modalities, and surgical techniques have enabled surgeons to address a wide spectrum of spinal pathology while sparing important soft tissue structures. The anatomic basis for a minimally invasive approach lies in the intramuscular plane (Wiltse plane) utilized to create the surgical working channel. This technique avoids having to transect or detach important paraspinal muscles (e.g., multifidus), which reduces the risk of muscle denervation and postoperative paraspinal muscle weakness.
Most published outcomes associated with MIS TLIF procedures are limited to retrospective series, and only a few prospective (non-randomized) mid to long-term (2-5 years) analyses (2,3). However, these initial reports have demonstrated that an MIS TLIF is associated with significantly less intraoperative blood loss, shorter hospitalization, early ambulation, and accelerated time to narcotic independence (4,5). More importantly, the fusion rates and clinical outcomes following an MIS TLIF are comparable to open TLIF procedures.
Similar to most minimally invasive techniques, there is a steep learning curve associated with MIS TLIF procedures (6). This technique requires the utilization of specialized instruments through a narrow and rigid working channel that is unfamiliar to most surgeons. In addition, MIS TLIFs rely on unconventional anatomical landmarks, intraoperative fluoroscopy, nerve monitoring, and surgical magnification (e.g., Microscope or Loops) to safely and effectively execute the surgical technique.
Fully understanding individual patient spinal anatomy and the intricacies of MIS instrumentation will likely help shorten the learning curve associated with this technically demanding procedure. Although further long-term prospective studies are warranted, an MIS TLIF procedure is a safe and effective alternative to open approaches for the management of lumbar spine pathology.
Acknowledgements
Disclosure: The authors declare no conflict of interest.
References
- Singh K. Minimally invasive transforaminal lumbar interbody fusion for lumbar spondylolisthesis. Asvide 2014;1:285. Available online: http://www.asvide.com/articles/298
- Kim JS, Jung B, Lee SH. Instrumented Minimally Invasive spinal-Transforaminal Lumbar Interbody Fusion (MIS TLIF); Minimum 5-years Follow-up With Clinical and Radiological Outcomes. J Spinal Disord Tech 2012. [Epub ahead of print].
- Wu RH, Fraser JF, Härtl R. Minimal access versus open transforaminal lumbar interbody fusion: meta-analysis of fusion rates. Spine (Phila Pa 1976) 2010;35:2273-81. [PubMed]
- Adogwa O, Parker SL, Bydon A, et al. Comparative effectiveness of minimally invasive versus open transforaminal lumbar interbody fusion: 2-year assessment of narcotic use, return to work, disability, and quality of life. J Spinal Disord Tech 2011;24:479-84. [PubMed]
- Parker SL, Adogwa O, Witham TF, et al. Post-operative infection after minimally invasive versus open transforaminal lumbar interbody fusion (TLIF): literature review and cost analysis. Minim Invasive Neurosurg 2011;54:33-7. [PubMed]
- Lee KH, Yeo W, Soeharno H, et al. Learning Curve of a Complex Surgical Technique: Minimally Invasive Transforaminal Lumbar Interbody Fusion (MIS TLIF). J Spinal Disord Tech 2014;27:E234-40. [PubMed]


