Moyamoya syndrome associated with Graves’ disease: a case series study
Introduction
Moyamoya disease is a cerebrovascular disorder characterized by bilateral progressive stenosis and occlusion of terminal portions of internal carotid artery (ICA) and accompanying typical net-like collateral vessels. The term “moyamoya syndrome” has been used to refer to cases that have angiographic features similar to those of moyamoya disease and causative systemic diseases. These known diseases include sickle cell disease, neurofibromatosis type 1, Down’s disease, cranial therapeutic irradiation and other rare diseases (1-3). Moyamoya syndrome associated with Graves’ disease has been rarely reported and the underlying coexisting mechanism remains unclear. Moreover, the relation between cerebral ischemic attack and thyroid function has garnered more interest for future stroke prevention in those patients.
The aim of this study is to identify the clinical and radiological findings of the patients admitted to Peking Union Medical College Hospital (PUMCH) with simultaneous diagnosis of moyamoya syndrome and Graves’ disease. We hypothesized that these patients will remain stable despite slow vascular progression until other clinical indices emerge. This theory also can explain why some patients with these two co-occurring conditions experienced ischemic/hemorrhagic attack and others did not. Possible mechanisms coexisting and predisposing these individuals to ischemic accidents are discussed.
Materials and methods
Patients and clinical data
We retrospectively and consecutively identified patients in PUMCH between May 2000 and December 2010. The patients who met the following criteria were included in the study sample: (I) they were diagnosed with Graves’ disease in an endocrinology clinic met full diagnostic criteria and had no evidence of atherosclerotic or other risk factors for cerebrovascular disease. Serum tests showed normal immune detection and homocysteine levels; (II) moyamoya vasculopathy involving the arteries around the circle of Willis was diagnosed by digital subtraction angiography (DSA) in three patients and magnetic resonance angiography (MRA) in nine patients.
Data collected included the clinical characteristics, predisposing conditions of cerebral vascular accident, prognosis, laboratory data, structure and vascular brain radiological measures. All patients provided written consent for use of lab, imaging and clinical information for research.
Neuroimaging protocol and reading
An magnetic resonance imaging (MRI) + MRA was performed on all patients, with two using 3-T high resolution MRI imaging for cross section of middle cerebral artery (MCA) and three patients given a DSA as well. All images were reviewed by a neurologist (J-N) and a radiologist (ML-L) for infarct and vascular lesions. The radiologist was blinded to the clinical aspects of the cases. In cases where diagnosis was uncertain, the supervising neurologist (LY-C) made the final determination.
Results
Demographics
All 12 patients were female and mean age at first presentation was 33.33±12.65 years (range was 11-55 years). One patient had a family history of Graves’ disease. No patient had any history of hypertension, hyperlipidemia or diabetic mellitus (DM).
Clinical presentations and prognosis
Eleven patients presented with acute ischemic stroke (n=9) and/or transient ischemic attack (TIA) (n=4) based on both clinical symptoms and brain magnetic resonance imaging/diffusion weighted image; only one patient reported dizziness. No patient had a hemorrhagic stroke. Symptoms associated with ischemic stroke/TIA included the hemiplegia, aphasia, numbness, facial droop and slurred speech. In addition, one patient presented with chorea on first onset [diffusion-weighted image (DWI) was negative at that time] and an ischemic attack on second onset. One patient presenting with chorea also had an accompanying TIA. All patients received anti-thyroid therapy and two had recurrent ischemic attack after six months of anti-thyroid drug withdrawal. Eleven patients received antiplatelet as well.
Ischemic lesions characteristics
MRI + DWI were performed in all patients within seven days of a stroke. Acute or subacute infarcts were found in nine patients based on DWI and apparent diffusion coefficient (ADC). Ischemic lesions were located in frontal lobar and/or watershed area in eight patients (Figure 1). Only one patient had an infarct in basal ganglion.
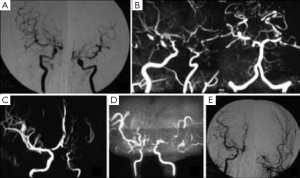
Cerebrovascular lesions
Stenosis or occlusion of bilateral terminal ICA and/or proximal anterior/middle cerebral arteries was found in nine patients with or without abnormal collateral vessels, three of those patients also presented with asymmetrical degree stenosis. Other three patients had unilateral terminal ICA stenosis and/or abnormal collateral vessels. Posterior cerebral artery (PCA) stenosis was found in two patients as well. Figure 1 shows variable vascular lesions presentations including bilateral symmetric or asymmetric and unilateral lesions. Two patients did high-resolution magnetic resonance (MR) for cross section of MCA and one of them did contrast MRI as well and showed normal MCA wall without plaque.
Laboratory data
Blood tests for immune detection (including the erythrocyte sedimentation rate, antinuclear antibody, extractable nuclear antibody, antineutrophil cytoplasmic antibody and anti-cardiolipin antibody) and homocysteine were performed for all patients and found to be normal. Interestingly, thyroid function tests demonstrated predominantly elevated thyroid hormone levels and suppressed thyroid stimulating hormone level consistent with a thyrotoxic state at the time in which all eleven patients had a stroke and/or TIA. Thyroid antibodies (including thyroid peroxidase antibody and thyroid gland antibody) were elevated level in all 12 patients. Ultrasonography showed no atherosclerotic plaque in bilateral internal carotid arteries and vertebral arteries.
Case illustration
One female patient first presented acute non-automatic movement when she was 15 years old. Rheumatic tests including erythrocyte sedimentation rate, C-reactive protein and Antistreptolysis-O test were all normal and negative for Sydenham’s chorea. Thyroid function tests identified elevated thyroxine and suppressed thyroid stimulating hormone levels at that time. MRI showed nothing remarkable, while the MRA findings of terminal ICA stenosis were consistent with moyamoya disease. She was discharged with a diagnosis of Moyamoya syndrome and Graves’ disease. She continued to take medication for her thyroid function. After withdrawal of treatment for six months, she experienced an acute onset stroke with concomitant increased T3 and T4 levels again when she was 21 years old. DWI showed a bright lesion with a dark ADC signal, indicating a subacute stroke in the left frontal lobe. MRA showed that the stenotic vessels were worse than previous. A high resolution 3-T MRI for MCA cross-section showed almost normal M1 segment of right MCA and no plaque presentation and occlusion of left MCA (Figure 2).
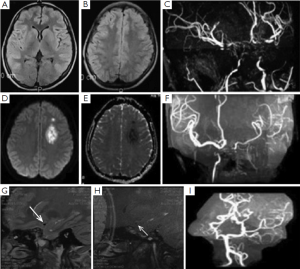
Discussion
There were several studies mentioned the relationship between moyamoya symdrome and Graves’ disease (4-8). Li et al. demonstrated that increased the thyroid function and auto-antibody levels are associated with moyamoya disease. This was confirmed by Kim et al., who also reported that elevated thyroid autoantibodies were frequently observed in patients with MMD (8). This evidence suggests potential correlation between these two diseases. Genetic and immunogenic backgrounds have been postulated to contribute to cerebrovascular lesion progression based on co-morbid familial moyamoya disease and high levels of thyroid antibody (4-8). In our study, new information was provided. High resolution 3-T MRI for MCA cross-section in two of our patients found no plaque or enhancement in the MCA wall, which was different from atherosclerotic features and vessel wall inflammation. Further studies are required to investigate the underlying heterogeneous mechanism.
Until now, less attention has been given to the relationship between thyroid function and vascular accidents in moyamoya syndrome with Graves’ disease. Based on the results from this study, all eleven patients with ischemic stroke and/or TIA were in a thyrotoxic state. Moreover, most of these patients fared well and had no recurrent of stroke with regular use of anti-thyroid drugs. The treatment of hyperthyroidism coincided with neurological improvement though the vascular lesions were presented. Two patients had a recurrent stroke after drug withdrawal for about six months. Previous published cases also reported a thyrotoxic state at the time of an acute clinical stroke (4,5). It is also known that cerebral hemodynamic changes occur in cases of thyrotoxic (4,5). We hypothesize cerebrovascular hemodynamic changes due to thyrotoxicosis might be responsible for ischemic attacks. A sudden surge of thyroid hormone levels should be avoided clinically to prevent cerebrovascular accident.
None of the 12 patients experienced a hemorrhagic stroke, which was consistent with previous reviews regarding moyamoya syndrome associated Graves’ disease (4,5). Bao et al. (9), reported that the most common initial symptom of adult moyamoya disease in China was a cerebral ischemic event. This is on the contrary to the earlier studies from Korea and Japan (10), which found adult moyamoya disease mostly presents with hemorrhagic stroke. More recent studies from Japan also supported that ischemic stroke is more common than hemorrhagic stroke in adult moyamoya disease (11). Currently there is no well-established medical treatment for moyamoya disease. While confirmations from future studies are warranted, it appears that antiplatelet or anticoagulant treatment is safe in our patients.
Moyamoya disease often presents with symmetrical bilateral vessel stenosis. However, cases with unilateral angiographic findings compatible with MMD have also been reported, and the term “unilateral moyamoya disease” has been in practical use. Previous studies identify that unilateral moyamoya disease account for 11-22% of all moyamoya patients (12-14). Also bilateral progression of these unilateral MMD has also been previously reported (13,15). Six patients (50%) in this study showed either asymmetrical or unilateral vessel lesions, which might give new insight into the different pathophysiological mechanism for the syndrome of Moyamoya disease with Graves’ disease. Clinically, the patients who present moyamoya-like asymmetrical vessel lesions should be further considered for thyroid function testing to identify the underlying etiology of vessel lesions and timely treatment of hyperthyroidism. In addition, two patients had posterior circulation stenosis. It has been noted that the posterior circulation serves as an important source of collateral blood supply in moyamoya disease. We speculate that the possible mechanism of these PCA lesions was long-term compensation for anterior circulation hypoperfusion.
Conclusions
Moyamoya syndrome associated Graves’ disease more commonly presented asymmetric stenosis or occlusion. Cerebrovascular hemodynamic changes due to thyrotoxicosis might contribute to the ischemic accidents. Future research is warranted to investigate whether traditional anti-thrombotic treatment is effective in prevention of ischemic attack.
Acknowledgements
We thank Dr. Rhoda Au for her help to edit the English writing.
Funding: This study was supported by Youth Foundation of Peking Union Medical College Hospital (Grant Number: 2010128).
Disclosure: The authors declare no conflict of interest.
References
- Hsu SW, Chaloupka JC, Fattal D. Rapidly progressive fatal bihemispheric infarction secondary to Moyamoya syndrome in association with Graves thyrotoxicosis. AJNR Am J Neuroradiol 2006;27:643-7. [PubMed]
- Smith ER, McClain CD, Heeney M, et al. Pial synangiosis in patients with moyamoya syndrome and sickle cell anemia: perioperative management and surgical outcome. Neurosurg Focus 2009;26:E10. [PubMed]
- Scott RM, Smith ER. Moyamoya disease and moyamoya syndrome. N Engl J Med 2009;360:1226-37. [PubMed]
- Ohba S, Nakagawa T, Murakami H. Concurrent Graves’ disease and intracranial arterial stenosis/occlusion: special considerations regarding the state of thyroid function, etiology, and treatment. Neurosurg Rev 2011;34:297-304; discussion 304. [PubMed]
- Malik S, Russman AN, Katramados AM, et al. Moyamoya syndrome associated with Graves' disease: a case report and review of the literature. J Stroke Cerebrovasc Dis 2011;20:528-36. [PubMed]
- Li D, Yang W, Xian P, et al. Coexistence of moyamoya and Graves’ diseases: the clinical characteristics and treatment effects of 21 Chinese patients. Clin Neurol Neurosurg 2013;115:1647-52. [PubMed]
- Li H, Zhang ZS, Dong ZN, et al. Increased thyroid function and elevated thyroid autoantibodies in pediatric patients with moyamoya disease: a case-control study. Stroke 2011;42:1138-9. [PubMed]
- Kim SJ, Heo KG, Shin HY, et al. Association of thyroid autoantibodies with moyamoya-type cerebrovascular disease: a prospective study. Stroke 2010;41:173-6. [PubMed]
- Bao XY, Duan L, Li DS, et al. Clinical features, surgical treatment and long-term outcome in adult patients with Moyamoya disease in China. Cerebrovasc Dis 2012;34:305-13. [PubMed]
- 10. Ikezaki K, Han DH, Kawano T, et al. A clinical comparison of definite moyamoya disease between South Korea and Japan. Stroke 1997;28:2513-7.
- Hoshino H, Izawa Y, Suzuki N, et al. Epidemiological features of moyamoya disease in Japan. Neurol Med Chir (Tokyo) 2012;52:295-8. [PubMed]
- Nagata S, Matsushima T, Morioka T, et al. Unilaterally symptomatic moyamoya disease in children: long-term follow-up of 20 patients. Neurosurgery 2006;59:830-6; discussion 836-7. [PubMed]
- Smith ER, Scott RM. Progression of disease in unilateral moyamoya syndrome. Neurosurg Focus 2008;24:E17. [PubMed]
- Kelly ME, Bell-Stephens TE, Marks MP, et al. Progression of unilateral moyamoya disease: A clinical series. Cerebrovasc Dis 2006;22:109-15. [PubMed]
- Kuriyama S, Kusaka Y, Fujimura M, et al. Prevalence and clinicoepidemiological features of moyamoya disease in Japan: findings from a nationwide epidemiological survey. Stroke 2008;39:42-7. [PubMed]


