RNA interference therapy: a new solution for intracranial atherosclerosis?
Atherosclerosis accounts for most kinds of cardiovascular disease, including myocardial ischemia, acute myocardial infarction, heartfailure, and stroke. It is a lifetime disease that often begins in childhood, but symptoms are not usually evident until middle age or later. According to the literature, aortic lesions are first evident with fatty streaks in the first decade and that coronary lesions become evident in the second decade and cerebral vessel involvement in the third or later (1). Intracranial atherosclerotic stenosis (ICAS) of major intracranial arteries reported to be a leading cause of ischemic stroke (2-4), which causes about 5-10% of strokes in white people, 15-29% of transient ischemic attacks or strokes in black people, and up to 30-50% of strokes in Asian people (5-7). Compared with other stroke subtypes, ICAS is associated with a higher risk of recurrent stroke (8). New therapeutic approaches to treat this high-risk disease include dual antiplatelet treatment, intensive management of risk factors, and endovascular therapy. Nevertheless, there are subgroups of patients who remain at high risk of stroke despite aggressive medical therapy. Further research is needed to identify these high-risk subgroups and to develop more effective treatments.
During recent decades there have been remarkable advances in biology, in which one of the most important discoveries is RNA interference (RNAi). RNAi is a post-transcriptional regulatory pathway that can result in efficient and specific silencing gene functions, which is a major advantage of targeted therapy. Comparably, the inhibitory effects of conventional pharmaceuticals are mainly achieved by blocking their targets’ function. However, some disease-related molecules, primarily proteins, do not have an enzymatic function or have a conformation that is hardly accessible to conventional drugs, therefore are considered as “non-druggable” targets. The newly-development RNAi approach can overcome the barrier and target the previous “non-druggable” targets by gene silencing (9). Efforts have been done to translate this new discovery into clinical applications for disease treatment. Currently, there are some ongoing or partially completed clinical trials of RNAi therapies in treating cancer, cardiovascular diseases, and eye disease etc. (10). In this review, we will briefly discuss the therapeutic potentials of gene silencing by RNAi in preventing stroke caused by ICAS, which may represent a promising direction in the future.
The pathophysiology of intracranial atherosclerosis
Atherosclerosis is a chronic inflammatory disease of the arterial wall resulting from a lipid dysfunction and a maladaptive inflammatory response. In the last few years, the pathology and pathogenesis of atherosclerosis in coronary arteries have been well studied due to its high incidence and its devastating effects on human beings (11). Atherosclerosis is initiated from endothelial dysfunction due to hypercholesterolaemia and inflammation, followed by smooth muscle proliferation and thickening of the arterial wall. The first atherosclerotic change in the artery is called a fatty streak, characterized by the adhesion of monocytes to the endothelium and their migration to subendothelial portions of the arterial wall. In this location, lipids accumulate intracellularly and develop a foamy appearance microscopically. The most common locations for the development of fatty streaks are vascular bifurcations or other areas with turbulent blood flow. As a person ages, the fatty streak is transformed into a fibrous plaque. It consists of a core of cellular debris, free extracellular lipid, and cholesterol crystals, lymphocytes, and connective tissue. The plaque grows insidiously over many years as a result of the elaboration of cytokines and factors released by endothelial cells, macrophages, platelets, and smooth muscle cells. Areas of calcification or hemorrhage also can occur within the plaque. Based on the knowledge from coronary arteries, in recent years the pathology of atherosclerosis in carotid arteries has become a hot topic, partly due to the researchers’ interest in the value of carotid endarterectomy in symptomatic and asymptomatic individuals with carotid artery stenosis (12). Carotid endarterectomy provides us with proper specimens of carotid artery to learn more about its morphology. However, intracranial vessels are not accessible to pathology sampling in live patients, which partly contributes to lacking of pathological knowledge of ICAS.
Traditional investigations of ICAS focus on the degree of arterial luminal stenosis. Digital subtraction angiography (DSA) is the only reliable standard in assessing luminal stenosis, which is an invasive procedure requiring injection of iodinated contrast. Other luminal angiography techniques such as computed tomography angiography (CTA) and magnetic resonance angiography (MRA) or transcranial Doppler (TCD) make it feasible to assess ICAS noninvasively. The focus on severity of stenosis has been reinforced because severe (70-99%) atherosclerotic stenosis was demonstrated as an independent predictor for stroke recurrence in the territory of the stenotic artery, with the risk of ~20% at 1 year, in the Warfarin versus Aspirin for Symptomatic Intracranial Disease (WASID) trial (3). However, the role of percent stenosis in predicting subsequent stroke risk has been superseded by collateral flow and hemodynamics in the same patient cohort (13). Thus, beyond luminal stenosis, many other features may reflect the characteristics of ICAS, such as plaque morphology and components, which might also be promising markers in risk stratification of patients with symptomatic ICAS (14).
High resolution magnetic resonance imaging (HRMRI) is a novel technique that is able to visualize vessel wall pathology (15-17). In patients with extracranial carotid stenosis, the components identified by HRMRI correlates well with pathological specimens (18), and also associated with stroke symptoms (16,19-23). In ICAS, Xu et al. compared vessel wall properties between symptomatic and asymptomatic atherosclerotic middle cerebral arteries (MCAs) and demonstrated that symptomatic MCA stenosis had a larger wall area, greater remodeling and higher prevalence of expansive remodeling (24). They also found that ipsilateral stroke is closely associated high signal on T1-weighted fat-suppressed images (HST1) within MCA plaque of HRMRI, which is highly suggested of fresh or recent intraplaque hemorrhage (25). Thus, the application of HRMRI in evaluating ICAS provides new insight into the vascular biology of plaque morphology and component, but lacking histological validation (18). To break through the limitation of “blind assessment” of ICAS in human, we performed a post-mortem study exploring the contributions of plaque characteristics to the occurrence of brain infarction (26). Seventy-six autopsy cases of Chinese cases aged 45 years were recruited and intracranial large arteries were removed for histological staining and evaluation. Our histological findings demonstrated that both luminal stenosis and plaque components (percentage of lipid area and presence of intraplaque neovasculature) may play a key role in leading to ischemic stroke (26), which for the first time provides direct histological evidence of plaque characteristics in causing brain infarction. The higher prevalence of intraplaque hemorrhage and neovasculature in symptomatic patients with MCA stenosis may provide a potential target for plaque stabilization. Further studies will be performed by using our acquired intracranial artery specimen to explore other cellular or molecular regulators, which may account for plaque instability.
Plaque neovascularization and hemorrhage: a potential target for plaque stabilization?
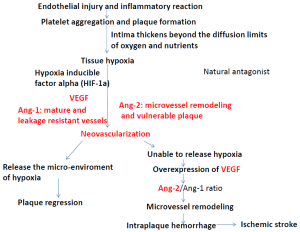
Angiogenesis or the growth of new blood vessels from existing host vessels is increasingly being recognized as important in the growth and progression of atherosclerosis. According to the literature (27), hypoxic conditions lead to upregulated expression of hypoxia inducible factor alpha (HIF-1α), a transcription factor, which promotes hypoxia dependent neovascularization in human atherosclerotic plaques. Vascular endothelial growth factor (VEGF) is a well-known regulator of angiogenesis and also closely related with neovascularization within atherosclerotic plaque (28,29). The Tie receptors, Tie1 and Tie2, and two ligands for Tie2, angiopoietin1 (Ang1) and Ang2 are also critical for vessel formation and maturation (30). The angiopoietins represent a relatively new family of related angiogenic growth factors, among which Ang1 and Ang2 are derived from mural cells and ECs, respectively and major regulators of angiogensis. Ang1 is known to stabilize nascent vessels and make them leak-resistant, presumably by facilitating communication between ECs and mural cells. Ang-2 is the natural antagonist of Ang-1 and its role appears to be contextual: (I) in the absence of VEGF, Ang2 acts as an antagonist of Ang1 and destabilizes vessels, ultimately leading to vessel regression; (II) in the presence of VEGF, Ang2 facilitates vascular sprouting. A high Ang2/Ang1 ratio has been found in vulnerable neo-vascularized plaques (31). Figure 1 shows the contributions of VEGF-Tie2 pathway in the process of atherosclerosis formation. Studies in atherosclerotic plaques removed from carotid endarterectomy demonstrated that overexpression of VEGF and its induced neovascularization may expedite the occurrence of ischemic symptoms (31,32). In ICAS, our previous post-mortem study also found that intraplaque hemorrhage and neovascularization might account for the occurrence of ischemic stroke (26,33). Intraplaque neovascularization is thought to be directly led to subsequent intraplaque hemorrhage (34,35), although further studies are required to delineate the causal relationship between neovascularization and intraplaque hemorrhage. Considering that intraplaque neovascularization and hemorrhage may predict subsequent ischemic event, strategies to “normalize” intraplaque neo-vessels to prevent the hemorrhage may be promising new avenues for prevention of ischemic stroke.
Similar strategies of anti-angiogenesis have been testing in tumor and eye disease. As stated, dysregulation of angiogenesis occurs in various pathologies and is one of the hallmarks of cancer. In tumors, the imbalanced regulation of pro- and anti-angiogenic signaling lead to an abnormal vascular network. With the discovery of VEGF as a major driver of angiogenesis, novel therapeutics aimed at inhibiting VEGF activity are developed to regress tumors by starvation. Preclinical studies have shown that anti-VEGF therapy changes tumor vasculature towards a more “mature” or “normal” phenotype (36). Besides in cancer, anti-VEGF agents such as ranibizumab and aflibercept are becoming increasingly well-established therapies and have replaced earlier approaches such as laser photocoagulation or photodynamic therapy in age-related macular degeneration (AMD). According to the previous experiences of anti-VEGF therapy in treating tumor and retinal disease, anti-VEGF or other anti-angiogenesis agents may provide a promising direction in preventing ischemic events due to atherosclerosis.
Plaque stabilization by targeted inhibiting neovascularization by RNAi
As we all know, atherosclerosis is a systemic chronic inflammatory disease, while angiogenesis is a physiological process in growth and development, as well as in wound healing. The potential beneficial effects of angiogenesis and its feature of generalization impede the systemic administration of anti-VEGF or other anti-angiogenesis agents. Thus, local targeted inhibition of intraplaque neovascularization is an optimal method that may meet our requirements. RNAi is defined as a mechanism of specific post-transcriptional gene-silencing mediated by small RNAs, including endogenous microRNA (miRNA) and exogenous small interfering RNA (siRNA) or short hairpin RNA (shRNA) (37). Double-stranded small RNAs incorporate into the RNA-induced silencing complex (RISC), where the strands are separated, and one strand guides RISC to the complementary or near-complementary region of target mRNA, suppressing the gene expression either by degrading mRNA or blocking mRNA translation (38,39).
For siRNA, it has a well-defined synthesized structure, a short (usually 21-bp) double-stranded RNA with phosphorylated 5' ends and hydroxylated 3' ends with two overhanging nucleotides. Since the half-life of siRNA is short, shRNA has been developed as an alternative RNA molecule. ShRNA is transcribed in the nucleus from an external expression vector bearing a short double stranded DNA sequence with a hairpin loop by RNA polymerase II or III. Comparing with siRNA, shRNA is constantly synthesized in host cells, leading to more durable gene silencing. Moreover, an shRNA expression vector cost less than the bulk manufacturing of siRNA (40). MiRNA is a class of small non-coding endogenous RNAs which play an important role in regulating cellular functions both physiologically and pathologically. Among three kinds of RNAi, shRNA is considered more potent in mediating gene silencing and more effective than either siRNA or miRNA (10).
In human tumors, angiogenesis is a key factor for neoplasia and tumor metastasis. The VEGF pathway is the hottest target of tumor angiogenesis (41). Thus, by selectively silencing VEGF pathway, RNAi technology has been used to inhibit angiogenesis. In a pancreatic tumor xenograft model, shRNAs against VEGF was intravenously and intratumorally delivered to inhibit cancer cell proliferation and tumor growth. The findings demonstrated down-regulated expression of VEGF-C mRNA and reduced microvessel density (MVD) in tumor, although shRNA showed a weaker inhibitory effect on tumor growth compared to the standard treatment for pancreatic cancer (42). Similar to VEGF, basic fibroblast growth factor (bFGF) is also an important pro-angiogenic growth factor. Another experiment indifferent pancreatic carcinoma cells, siRNA against bFGF reduced the amount of bFGF mRNA and inhibited endostatin secretion (43). Thus, the experiences in tumor therapy demonstrate that antiangiogenic treatment can be developed as therapeutic targets for angiogenesis-related disease. Based on recent pathological knowledge of ICAS and its molecular regulators, we will explore more and more target gene for RNAi therapy.
Local delivery of siRNA by nanoparticle carrier
RNAi delivery systems consist of viral delivery systems and non-viral delivery systems (chemical modification, liposomes and nanoparticles). Nanoparticles are particulate dispersion or solid particles with a size of 10-100 nm. A polymeric nanoparticle for gene delivery is considered more stable with larger capacity as nanosized entities enable a very limited volume to provide an enormous surface area for transport, chemical reactions, and interaction with biological systems. Compared to traditional delivery system, the unique advantage of nanoparticles involving metallic core is its capability to study in vivo distribution of siRNAs by using magnetic resonance imaging (MRI) (10). Advances in biomedical imaging allow the study of plaque-targeting nanoparticles in a dynamic fashion (44). At the same time, nanoparticle delivery system can be modified for targeting specific cells or molecules. We hypothesize that RNAi therapy delivered by modified nanoparticle carrier can achieve in vivo imaging and anti-angiogenesis within atherosclerotic plaques, which may help to evaluate plaque morphology and to stabilize plaques at a high risk of rupture. With the rapid developments in studies about therapeutic and diagnostic nanomaterials, future studies further exploring the molecular biology of atherosclerosis may provide more drug targets for plaque stabilization.
Acknowledgements
Disclosure: The authors declare no conflict of interest.
References
- Moossy J. Cerebral infarcts and the lesions of intracranial and extracranial atherosclerosis. Arch Neurol 1966;14:124-8. [PubMed]
- Chimowitz MI, Lynn MJ, Howlett-Smith H, et al. Comparison of warfarin and aspirin for symptomatic intracranial arterial stenosis. N Engl J Med 2005;352:1305-16. [PubMed]
- Kasner SE, Chimowitz MI, Lynn MJ, et al. Predictors of ischemic stroke in the territory of a symptomatic intracranial arterial stenosis. Circulation 2006;113:555-63. [PubMed]
- Wong KS, Li H, Chan YL, et al. Use of transcranial Doppler ultrasound to predict outcome in patients with intracranial large-artery occlusive disease. Stroke 2000;31:2641-7. [PubMed]
- Gorelick PB, Wong KS, Bae HJ, et al. Large artery intracranial occlusive disease: a large worldwide burden but a relatively neglected frontier. Stroke 2008;39:2396-9. [PubMed]
- Sacco RL, Kargman DE, Gu Q, et al. Race-ethnicity and determinants of intracranial atherosclerotic cerebral infarction. The Northern Manhattan Stroke Study. Stroke 1995;26:14-20. [PubMed]
- Wong LK. Global burden of intracranial atherosclerosis. Int J Stroke 2006;1:158-9. [PubMed]
- Holmstedt CA, Turan TN, Chimowitz MI. Atherosclerotic intracranial arterial stenosis: risk factors, diagnosis, and treatment. Lancet Neurol 2013;12:1106-14. [PubMed]
- Daka A, Peer D. RNAi-based nanomedicines for targeted personalized therapy. Adv Drug Deliv Rev 2012;64:1508-21. [PubMed]
- Deng Y, Wang CC, Choy KW, et al. Therapeutic potentials of gene silencing by RNA interference: principles, challenges, and new strategies. Gene 2014;538:217-27. [PubMed]
- Virmani R, Kolodgie FD, Burke AP, et al. Lessons from sudden coronary death: a comprehensive morphological classification scheme for atherosclerotic lesions. Arterioscler Thromb Vasc Biol 2000;20:1262-75. [PubMed]
- Benavente O, Moher D, Pham B. Carotid endarterectomy for asymptomatic carotid stenosis: a meta-analysis. BMJ 1998;317:1477-80. [PubMed]
- Liebeskind DS, Cotsonis GA, Saver JL, et al. Collaterals dramatically alter stroke risk in intracranial atherosclerosis. Ann Neurol 2011;69:963-74. [PubMed]
- Bodle JD, Feldmann E, Swartz RH, et al. High-resolution magnetic resonance imaging: an emerging tool for evaluating intracranial arterial disease. Stroke 2013;44:287-92. [PubMed]
- Yuan C, Kerwin WS, Ferguson MS, et al. Contrast-enhanced high resolution MRI for atherosclerotic carotid artery tissue characterization. J Magn Reson Imaging 2002;15:62-7. [PubMed]
- Chu B, Kampschulte A, Ferguson MS, et al. Hemorrhage in the atherosclerotic carotid plaque: a high-resolution MRI study. Stroke 2004;35:1079-84. [PubMed]
- Kang X, Polissar NL, Han C, et al. Analysis of the measurement precision of arterial lumen and wall areas using high-resolution MRI. Magn Reson Med 2000;44:968-72. [PubMed]
- Xu WH, Li ML, Gao S, et al. Middle cerebral artery intraplaque hemorrhage: prevalence and clinical relevance. Ann Neurol 2012;71:195-8. [PubMed]
- Zhao XQ, Dong L, Hatsukami T, et al. MR imaging of carotid plaque composition during lipid-lowering therapy a prospective assessment of effect and time course. JACC Cardiovasc Imaging 2011;4:977-86. [PubMed]
- Moody AR, Murphy RE, Morgan PS, et al. Characterization of complicated carotid plaque with magnetic resonance direct thrombus imaging in patients with cerebral ischemia. Circulation 2003;107:3047-52. [PubMed]
- U-King-Im JM, Tang TY, Patterson A, et al. Characterisation of carotid atheroma in symptomatic and asymptomatic patients using high resolution MRI. J Neurol Neurosurg Psychiatry 2008;79:905-12. [PubMed]
- Maldonado N, Kelly-Arnold A, Vengrenyuk Y, et al. A mechanistic analysis of the role of microcalcifications in atherosclerotic plaque stability: potential implications for plaque rupture. Am J Physiol Heart Circ Physiol 2012;303:H619-28. [PubMed]
- Makowski MR, Botnar RM. MR imaging of the arterial vessel wall: molecular imaging from bench to bedside. Radiology 2013;269:34-51. [PubMed]
- Xu WH, Li ML, Gao S, et al. In vivo high-resolution MR imaging of symptomatic and asymptomatic middle cerebral artery atherosclerotic stenosis. Atherosclerosis 2010;212:507-11. [PubMed]
- Altaf N, MacSweeney ST, Gladman J, et al. Carotid intraplaque hemorrhage predicts recurrent symptoms in patients with high-grade carotid stenosis. Stroke 2007;38:1633-5. [PubMed]
- Chen XY, Wong KS, Lam WW, et al. Middle cerebral artery atherosclerosis: histological comparison between plaques associated with and not associated with infarct in a postmortem study. Cerebrovasc Dis 2008;25:74-80. [PubMed]
- Sluimer JC, Gasc JM, van Wanroij JL, et al. Hypoxia, hypoxia-inducible transcription factor, and macrophages in human atherosclerotic plaques are correlated with intraplaque angiogenesis. J Am Coll Cardiol 2008;51:1258-65. [PubMed]
- Costa PZ, Soares R. Neovascularization in diabetes and its complications. Unraveling the angiogenic paradox. Life Sci 2013;92:1037-45. [PubMed]
- Lam MK, Al-Ansari S, van Dam GM, et al. Single-chain VEGF/Cy5.5 targeting vegf receptors to indicate atherosclerotic plaque instability. Mol Imaging Biol 2013;15:250-61. [PubMed]
- Finn AV, Jain RK. Coronary plaque neovascularization and hemorrhage: a potential target for plaque stabilization? JACC Cardiovasc Imaging 2010;3:41-4. [PubMed]
- Post S, Peeters W, Busser E, et al. Balance between angiopoietin-1 and angiopoietin-2 is in favor of angiopoietin-2 in atherosclerotic plaques with high microvessel density. J Vasc Res 2008;45:244-50. [PubMed]
- Hiyama T, Tanaka T, Endo S, et al. Angiogenesis in atherosclerotic plaque obtained from carotid endarterectomy: association between symptomatology and plaque morphology. Neurol Med Chir (Tokyo) 2010;50:1056-61. [PubMed]
- Chen XY, Wong KS, Lam WW, et al. High signal on T1 sequence of magnetic resonance imaging confirmed to be intraplaque haemorrhage by histology in middle cerebral artery. Int J Stroke 2014;9:E19. [PubMed]
- Fleiner M, Kummer M, Mirlacher M, et al. Arterial neovascularization and inflammation in vulnerable patients: early and late signs of symptomatic atherosclerosis. Circulation 2004;110:2843-50. [PubMed]
- Kolodgie FD, Gold HK, Burke AP, et al. Intraplaque hemorrhage and progression of coronary atheroma. N Engl J Med 2003;349:2316-25. [PubMed]
- Goel S, Duda DG, Xu L, et al. Normalization of the vasculature for treatment of cancer and other diseases. Physiol Rev 2011;91:1071-121. [PubMed]
- Wang Z, Rao DD, Senzer N, et al. RNA interference and cancer therapy. Pharm Res 2011;28:2983-95. [PubMed]
- Zamore PD, Tuschl T, Sharp PA, et al. RNAi: double-stranded RNA directs the ATP-dependent cleavage of mRNA at 21 to 23 nucleotide intervals. Cell 2000;101:25-33. [PubMed]
- Martinez J, Patkaniowska A, Urlaub H, et al. Single-stranded antisense siRNAs guide target RNA cleavage in RNAi. Cell 2002;110:563-74. [PubMed]
- Aagaard L, Rossi JJ. RNAi therapeutics: principles, prospects and challenges. Adv Drug Deliv Rev 2007;59:75-86. [PubMed]
- Fakih M. The evolving role of VEGF-targeted therapies in the treatment of metastatic colorectal cancer. Expert Rev Anticancer Ther 2013;13:427-38. [PubMed]
- Shi Y, Tong M, Wu Y, et al. VEGF-C ShRNA inhibits pancreatic cancer growth and lymphangiogenesis in an orthotopic fluorescent nude mouse model. Anticancer Res 2013;33:409-17. [PubMed]
- Yan C, Wang C, Dong M, et al. RNA interference-mediated silencing of VEGF and bFGF suppresses endostatin secretion in pancreatic carcinoma cells. Oncol Lett 2013;5:1031-5. [PubMed]
- Kim Y, Lobatto ME, Kawahara T, et al. Probing nanoparticle translocation across the permeable endothelium in experimental atherosclerosis. Proc Natl Acad Sci U S A 2014;111:1078-83. [PubMed]


