Chemically defined serum-free and xeno-free media for multiple cell lineages
Introduction
Cell and tissue culture has become an indispensable research tool, and serum is commonly used as a supplement to cell culture media. Serum provides a broad spectrum of macromolecules, proteins, attachment and spreading factors, low molecular weight nutrients, hormones, and growth factors. The most commonly used animal serum supplement is fetal bovine serum (FBS) and fetal calf serum (FCS). A major drawback from the use of serum is the wide range of possible contaminants. Protein concentration found in media containing 10% serum has a range of 6,200-10,000 mg/L, where the concentration for a defined recombinant protein produced in mammalian cells ranges from a few mg/L to 1,000 mg/L (1). It is reported that native protein levels of accumulation can be even lower. If the protein is functionally related to a serum protein it may be impossible to separate them, making the serum proteins a huge contaminant. Such contamination could be catastrophic for the production of a protein-based drug, as well as problematic for researchers trying to study the mechanisms of a specific protein. Experimental results can be difficult to recapitulate using serum media because of the variation between serum lots; and the methods used to obtain the serum are ethically questioned. Serum-free and animal free (xeno) medium presents an alternative to serum-containing media for the cultivation of cells.
Growth media can be categorized into serum-based, serum-free, and chemically defined animal (xeno) and serum-free. True serum-based media are the most complex compositions, with the major reason for omitting serum from culture media being the fact that serum is a supplement of unknown composition that could be contaminated with unwanted factors (2). There is an obvious difference between serum-free media and chemically defined media. Serum-free media may contain undefined animal-derived products such as serum albumin (purified from blood), hormones, growth factors, various proteins, etc (2,3). Undefined animal-derived products contain lipids contents of albumin which is considered to be a contaminant (2,3). In sharp contrast, chemically defined media requires that all of the components be identified, as well as their concentrations. Therefore a chemically defined medium must be completely free from serum, animal-derived or human, as well as albumin free which is commonly reintroduced as a separate ingredient, allowing the product to still be deceptively labeled as serum-free (2).
Most commercial formulations for neuronal culture are expensive and their true contents are proprietary [Neurobasal Media, Life Technologies Inc., Neuro Cult-Xeno Free (XF) medium, Stem Cell Technologies, Inc.]. Some of these formulations either contain serum or recommend the addition of serum to promote cellular proliferation. We have adopted a standard DMEM/F12 base and developed a media that maintains various neuronal cell types. Our formulation does not include any human or bovine serum, albumin, nor does it include any other animal products. As a result of the media’s ability to maintain and differentiate neurons and glial cells it was given the name Neuro-Pure, however it was tested and shown to support additional cell lines such fibroblast and multiple cancer cell lines. In this paper we report the evaluation of commercially available neuronal media [Neuro-PureTM, Neurobasal A media (Life Technologies), and XF Neuro Cult media (Stem Cell Technologies)] to support the growth of various cell culture lines with the goal evaluating the necessity of serum. We report Neuro-Pure to be a more cost-effective (Table 1), XF and serum free alternative media for maintaining neuronal, fibroblast and specific cancer cell lineages.
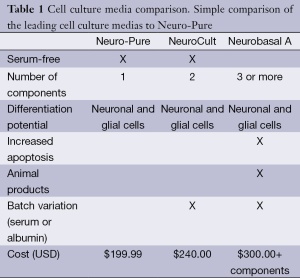
Full table
Materials and methods
Tissue culture for glioma derived CSCs, hNP1TM and other cell lines
In brief, a patient suffering with a high grade (grade IV) glioblastoma tumor of the brain, located in the cerebellum. The tumor was approximately 3 cm in diameter and use of this tumor was approved by the Committee on Human Research of the Winship Cancer Center at Emory University (Atlanta, GA, USA), and all patients signed an approved consent document prior to surgery. The specimen was examined by a neuropathologist to verify that each case met the criteria for GBM. The specimen was cut into 3 fragments and washed with Hanks’s balanced salt solution (Mediatech) and enzymatically digested. Sample is centrifuge at 1,500 for 5 min 2×. Discard supernatant and resuspend cells in media. Place cells into low binding T25 flasks @ 2,500-5,000 cells per cm2. Every two days 1 mL of fresh media plus growth factors. hEGF (20 ng/mL); hFGF-b (10 ng/mL); and heparin (2 µg/mL) was added for 7-14 days, when the neurospheres were 200-500 µM in diameter. Neurospheres were mechanically dissociated and harvested or passaged for continual growth. Neurospheres that were differentiated were placed on 12 well plates coated with polyornithine (Sigma; 20 µg) and laminin (Sigma; 10 µg). After 24 hrs media was replaced with growth factor free media and allowed to differentiate up to 12 days. Samples were harvested at days 1 and 12. All other cell types written about were commercially purchased from ATCC and were used according to their suggested protocols for maintenance and differentiation.
hNP1 Neural Progenitor cells (hNPC’s) (ArunA Biomedical) derived from WA09 hESCs were cultured in Matrigel™ (BD Bioscience) coated tissue culture dishes. Cells were maintained in complete neural expansion medium composed of AB2™ medium supplemented with 1× ANS™ (ArunA Biomedical Inc.), 20 ng/mL Leukemia Inhibitory Factor (LIF, Millipore), 2 mM L-Glutamine, 0.5 U/mL penicillin, 0.5 U/mL streptomycin (both from Invitrogen), 20 ng/mL basal human fibroblast growth factor (bFGF, Millipore) at 37 °C and 5% CO2. Culture medium was changed every other day and hNP1 cells were passaged every 3-4 days using either a cell scraper or manual pipetting.
Neuro-Pure (Jeevan Biosciences, Inc.), Neurobasal A (Life Technologies), and Neuro Cult-XF (Stem Cell Technologies) media were purchased and prepared according to the manufacturers instructions. L-Glutamine was added to Neurobasal A, Neuro Cult-XF media and Neuro-Pure. No additional components were added to the Neuro-Pure media unless specified.
Trypan blue cell counting
Cells were plated on 24-well flat bottom plates purchased from Corning, and grown for three passages (p3). After several repeats, cells were plated at the best individual density that did not allow for over growth before cells could be removed for counting. A549 cell were plated at 2×104, MCF7 cells were at 1×105, Hs578T cells were plated at 5×104, MEFs were plated at 2×104, and HFFs were plated at 5×104. Cells were grown in triplicate, and in the indicated medias for 48 hrs and removed from the plate using Accutase. Cells were counted using a hemocytometer and trypan blue (1:2) and final numbers were compared statistically by performing an ANOVA.
MTT assay
Cells were harvested by centrifugation. Adherent cells were released by Accutase or scraping. Washed (PBS) cells were serially diluted in culture medium from 1×106 to 1×103 cells per mL in triplicate, including three control wells of medium alone to provide the blanks for absorbance readings. Cells were incubated under conditions appropriate for the cell line for 6 to 48 hours (to recover from handling). The time required will vary but 12 hours to overnight is sufficient for most cell types. MTT reagent was added to each well, including controls. Plate was returned to cell culture incubator for 2 to 4 hours. Periodically cells were viewed under an inverted microscope for presence of intracellular punctate purple precipitate. When the purple precipitate is clearly visible under the microscope detergent reagent was added to all wells, including controls. Plate with cover was placed in the dark for 2 to 4 hours or overnight at room temperature. Plate cover was removed and the absorbance in each well was measured, including the blanks, at 570 nm in a microtiter plate reader. Absorbances can be read with any filter in the wavelength range of 550-600 nm. The reference wavelength should be higher than 650 nm. The blanks should give values close to zero (±0.1). If the readings are low return the plate to the dark for longer incubation.
Neurosphere formation and differentiation
Neurospheres (CSCs and U87 cells) formed in low attachments flasks and were grown in suspension in 3% O2/5% CO2 for 7 days in the indicated media. Neurospheres were carefully removed from low-binding tissue culture grade plates and re-plated and allowed to adhere onto polyornithine (Sigma-Aldrich) and laminin (10 µg) (Sigma-Aldrich) coated 10 cm tissue culture plates (Corning). Neurospheres were differentiated for 7 days in the indicated media without growth factors.
Immunocytochemistry
An assessment of differentiation and apoptosis was performed using immunocytochemistry. Antibodies selected to identify states of cellular differentiation include GFAP, O4, Oct-4, Tra-1 and TUJ-1 (Cell Signaling), as well as antibody for activated caspase-3 (Cell Signaling) as an indicator or apoptosis. Differentiated neurospheres were fixed with 2% paraformaldehyde (PFA) for 5 minutes, washed 2× in PBS and permeated with 0.5% Triton-X-100 for five minutes. After washing 2× in PBS, cells were labeled with antibodies against GFAP and TUJ-1 (Cell Signaling) and DAPI (KPL). Images were taken and scored positive using the Nikon Eclipse Inverted Microscope System.
Neurosphere area and neurite length measurement
Neurosphere area and neurite length were determined using NIS-Elements imaging software (Nikon). The area is the count of pixels detected in an object (computed by NIS-Elements imaging software). Neurite length was calculated as the distance between two points (computed by NIS-Elements imaging software).
Statistical analysis
Data are expressed as mean + standard deviation (SD). Statistical comparison between groups using an ANOVA, with P value
Results
Neuronal cell proliferation in the absence of serum
As mentioned a XF, serum-free and albumin-free growth media can maintain multiple cell lines which include neuronal, fibroblast, and cancer cells; as well as primary cancer stem cells derived from glioblastoma (4,5). To compare the affects each media has on the characteristics of cell growth and differentiation, we used a primary cancer stem cell (CTB-1) (4), a neuronal stem cell-like cell [U87 (ATCC)] and a neuronal progenitor cell line [hNP1™ (ArunA Biomedical)]. The three cell lines were maintained and differentiated in Neuro-Pure, Neurobasal A (supplemented with N2 and B27), and Neuro Cult-XF media. Neurosphere formation using CTB-1 and U87 cells was achieved using all three medias (Figure 1). The diameter of the neurospheres and neurite formation during differentiation measured greater in the population of cells grown in the Neurobasal A serum media, however, cell populations grown in NeuroCult-XF and Neuro-Pure produced a substantial number of neurospheres (Figure 2A-D). It has been reported that the presence serum has the ability to alter cell growth cycle as well as increase the rate of proliferation and differentiation (4-12). Serum accelerated proliferation and differentiation produces more mature non-dividing neurons that fail to receive appropriate input from the target field, resulting in apoptosis (6-14). We observed the formation of more activated caspase-3 in cells grown in Neurobasal A media (Figure 2E) confirming the aforementioned, that serum components specifically albumin, can induce apoptosis. Likewise, neuronal progenitor cells grown in Neuro-PureTM demonstrated a more controlled and symmetric pattern of differentiation versus NeuroCult-XF and Neurobasal A (supplemented with N2 and B27), which contains animal products and serum. In total these results show that growth media containing serum and/or albumin as well as other animal products are not required to grow and differentiate CSCs, U87, neuronal progenitor cells (Figure 1), and potentially countless other cells of neuronal origin.
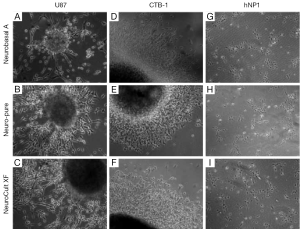
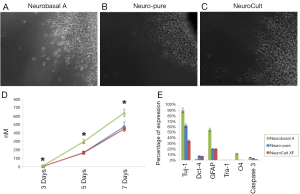
Differentiation and apoptosis in the absence of serum
Glioma derived CSCs were grown and differentiated in each respective media. As shown in Figure 2E, all cells show signs of differentiation in the absences of growth factors. Both XF medias (Neuro-Pure and NeuroCult) produced GFAP positive cells (Astrocytes) equally with regards to total cell population. However, Neurobasal A produced a more than twice the number of Astrocytes that were produced in the XF and serum-free medias. Furthermore, less than 1% of the cells differentiated in Neuro-Pure and NeuroCult were positive for O4, a marker that is present on early Oligodendrocytes. Neurobasal A produced a population of 10% that were positive for O4 surface marker. Neurobasal A produced more Tuj-1 positive cells (90%) than Neuro-Pure (60%) and NeuroCult (35%), however all medias were indistinguishable with regards to Tra-1 expression on their respective populations differentiated CSCs. Positive activated (cleaved) caspase-3 is an accepted marker of apoptosis. Neurobasal A produced more than 2-fold the number of positive cells in comparison to Neuro-Pure and NeuroCult. This data suggest Neurobasal A produces more differentiated cells of various neuronal lineages; however these cells are more apoptotic and less pluripotent than cells grown in the XF media (Neuro-Pure and NeuroCult). Additionally, Neurobasal A grown cells appear to have 2-3-fold less cells that are Oct-4 positive, an indicator of stemness or pluripotency (less differentiation). This suggests more spontaneous differentiation occurs with the use of Neurobasal A.
Cancer cell line and fibroblast proliferation in the absence of serum
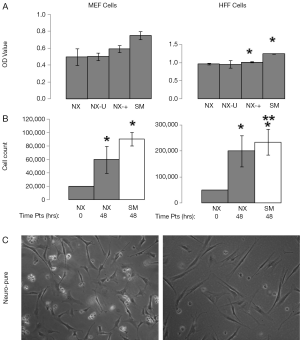
Some of the most commonly used and published cancer cell lines include A549, HeLa, 143B, MCF7, and HS578T, and are typically maintained in media containing serum. Using Neuro-Pure as a growth media, we sought to determine if serum was required to maintain these cancer lines. The following specific conditions were chosen for Neuro-Pure, (I) as manufactured without adding any factors (NX); (II) unrefrigerated but stored in the absence of light for 2 weeks (NX-U); (III) with the addition of serum (10% FBS) (NX-+), and the manufacturers recommended or suggested media for each cell type which is a DMEM/F12 base plus 10% FBS (SM). Neuro-Pure, without additional factors, supported growth in A549, MCF7, and HS578T cancer cell lines (Figure 3). Likewise unrefrigerated Neuro-Pure (NX-U) produced results that are similar to those seen in normal Neuro-Pure (NX), which confirms the media’s stability in the absence of serum (Figure 3). When Neuro-Pure was compared to Neuro-Pure with serum (NX-+) and the manufacturer’s suggested media plus serum (SM) all cell lines proliferated at equal rates except HS587T cells which demonstrated enhanced proliferation in the presence of serum (Figure 3). Likewise with mouse embryonic fibroblasts (MEFs), no statistically significant difference was observed amongst all four media (Figure 4), however human forskin fibroblasts (HFFs) showed a significant increase of growth in the presence of serum (Figure 4). All cells showed significant increase in growth after 48 hours in Neuro-Pure, as indicated by the cell count performed using trypan blue (Figures 3,4). These results confirm that chemically defined media efficiently sustains cell growth and is a viable alternative to serum containing products.
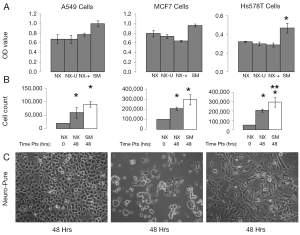
Discussion
Cell culture is commonly used in laboratory settings and is of great use in toxicology and drug testing.
Serum-free media presents an alternative to serum-containing media and offers several advantages, which include better understanding of media composition, reduced cost, and a reduced risk of contamination by infectious agents found in serum. There is an increasing use of serum-free, animal-free, and protein-free medias; this is particularly true in the biopharmaceutical industry, where the use of serum is considered to be a potential health hazard as well as a source of unwanted contamination. Serum-free media are typically without animal or human serum, but it may contain serum constituents such as albumin which are added in many cases as supplements. In some cases albumin has been shown to promote brain development by triggering the synthesis and release of oleic acid by astrocytes (13), as well as glutamate (7), which induces neuronal differentiation (13). However, albumin can be added to media very inexpensively, without the complications associated with adding total animal serum which contains growth factors, steroids, and biologically active anti-inflammatory drugs (15). We show neuronal cells can be grown and differentiated without serum or albumin, and in the absence of animal products.
Cancer is defined medically as a malignant neoplasm and encompasses a broad group of diseases. In cancer, cells divide and grow uncontrollably, forming malignant tumors, invading neighboring tissues or to more distant parts of the body through the lymphatic system or bloodstream. As shown previously (4), Neuro-Pure was used to grow glioma derived CSC and showed tumorigenicity both in vitro and in vivo. CSCs formed neurospheres while grown in Neuro-Pure (in vitro) and were subsequently placed in the flank of a mouse. These cells migrated to the brain and formed tumor, confirming tumorigenicity in vivo. The absence of serum did not produce results that were different from the original in situ state. It is difficult to identify growth factors and media that universally maintains the over 200 different known cancers that affect humans, and the over one thousand cancer cell lines that are used and maintained in laboratory settings. As stated previously, serum is a common contaminant that is used to maintain cells, including cancer cells. Serum and all of its factors fuel growth in cancer stem cells; however, it has been shown it is not required in some cancer lines (16-18). Although we did not test the entire cancer cell library, in this manuscript we show several cancer cell lines, which are commonly used, do not require serum to be maintained and differentiated.
Similarly fibroblast and other cells have been used to develop low serum-media systems for drug testing. Fibroblasts are also a source for reprogramming cells (iPSCs). These cells derived from various disease populations have great potential as models for drug development; however, serum of any source poses a potential threat to reprogramming, maintenance, and differentiation of cells. Here we show fibroblast of human and mouse source can be maintained and proliferated without serum, in addition to previous reports where we grew neurons and astrocytes in Neuro-Pure, without serum. In the near future we will test other cells such as iPSCs for their ability to be maintained and differentiated in serum free media.
Conclusions
To conclude, Neuro-Pure media and protocols represent a cost-effective and reproducible alternative for the maintenance and differentiation of several cell types including primary cancer stem cells derived from glioma. Additionally, neither serum nor albumin is required components which extend the shelf life of the media as well as increasing experimental reproducibility. Completely eliminating serum promotes the establishment of standardized protocols that conform to good cell culture practices which is of high precedence in this era with a promise of cell-based therapy and regenerative medicine.
Acknowledgements
We thank Saju Samuel (COO; Jeevan Biosciences, Inc.) for administrative support of this project. We also thank Donovan Moxey, Ph.D for consulting services (Jeevan Biosciences, Inc.)-IANAFS.
Disclosure: The authors declare no conflict of interest.
References
- Monterey MD, Szerlip NJ, Mathupala SP. Low-cost media formulation for culture of brain tumor spheroids (neurospheres). Biotechniques 2013;55:83-8. [PubMed]
- Broedel SE Jr, Papciak SM. The Case for Serum-Free Media. BioProcess Int 2003;1:56-8.
- Yao S, Chen S, Clark J, et al. Long-term self-renewal and directed differentiation of human embryonic stem cells in chemically defined conditions. Proc Natl Acad Sci U S A 2006;103:6907-12. [PubMed]
- Tucker-Burden C, Chappa P, Krishnamoorthy M, et al. Lectins identify glycan biomarkers on glioblastoma-derived cancer stem cells. Stem Cells Dev 2012;21:2374-86. [PubMed]
- Krishnamoorthy M, Gerwe BA, Scharer CD, et al. Ethanol alters proliferation and differentiation of normal and chromosomally abnormal human embryonic stem cell-derived neurospheres. Birth Defects Res B Dev Reprod Toxicol 2013;98:283-95. [PubMed]
- Byun K, Bayarsaikhan E, Kim D, et al. Activated microglial cells synthesize and secrete AGE-albumin. Anat Cell Biol 2012;45:47-52. [PubMed]
- Tabernero A, Granda B, Medina A, et al. Albumin promotes neuronal survival by increasing the synthesis and release of glutamate. J Neurochem 2002;81:881-91. [PubMed]
- Okamura K, Dummer P, Kopp J, et al. Endocytosis of albumin by podocytes elicits an inflammatory response and induces apoptotic cell death. PLoS One 2013;8:e54817. [PubMed]
- Pisciotta A, Riccio M, Carnevale G, et al. Human serum promotes osteogenic differentiation of human dental pulp stem cells in vitro and in vivo. PLoS One 2012;7:e50542. [PubMed]
- Chua KH, Wan Safwani WK, Chin SS, et al. Human serum promotes the proliferation but not the stemness genes expression of human adipose-derived stem cells. BBiotechnol Bioprocess Eng 2012;17:1306-13.
- Phadnis SM, Joglekar MV, Venkateshan V, et al. Human umbilical cord blood serum promotes growth, proliferation, as well as differentiation of human bone marrow-derived progenitor cells. In Vitro Cell Dev Biol Anim 2006;42:283-6. [PubMed]
- Byun K, Bayarsaikhan E, Kim D, et al. Induction of neuronal death by microglial AGE-albumin: implications for Alzheimer’s disease. PLoS One 2012;7:e37917. [PubMed]
- Tabernero A, Lavado EM, Granda B, et al. Neuronal differentiation is triggered by oleic acid synthesized and released by astrocytes. J Neurochem 2001;79:606-16. [PubMed]
- Li X, Pabla N, Wei Q, et al. PKC-delta promotes renal tubular cell apoptosis associated with proteinuria. J Am Soc Nephrol 2010;21:1115-24. [PubMed]
- Vinci F, Fabbrocino S, Fiori M, et al. Determination of fourteen non-steroidal anti-inflammatory drugs in animal serum and plasma by liquid chromatography/mass spectrometry. Rapid Commun Mass Spectrom 2006;20:3412-20. [PubMed]
- Allegra JC, Lippman ME. Growth of a human breast cancer cell line in serum-free hormone-supplemented medium. Cancer Res 1978;38:3823-9. [PubMed]
- Kaighn ME, Kirk D, Szalay M, et al. Growth control of prostatic carcinoma cells in serum-free media: interrelationship of hormone response, cell density, and nutrient media. Proc Natl Acad Sci U S A 1981;78:5673-6. [PubMed]
- Liang C, Chen W, Zhi X, et al. Serotonin promotes the proliferation of serum-deprived hepatocellular carcinoma cells via upregulation of FOXO3a. Mol Cancer 2013;12:14. [PubMed]


