Recent advances in the development of anti-HER2 antibodies and antibody-drug conjugates
Background and scope of the problem
Approximately 20% of breast cancers are characterized by amplification of the human epidermal growth factor receptor 2 (HER2) gene and overexpression of HER2, which functions as a driver oncogene for such tumors (1,2). Overexpression of HER2 leads to increased signal transduction and activation of the MAPK and PI3K/Akt pathways (3). HER2-positive breast cancers have historically been associated with decreased relapse free survival, decreased overall survival (OS) and poorer prognosis (1,4-6). In contrast to normal cells which express approximately 20,000 HER2 receptors on the cell membrane, each HER2-positive breast cancer cell expresses approximately one to two million HER2 receptors, making HER2 an attractive candidate for targeted therapy (7). Indeed, trastuzumab (Herceptin, Genentech, South San Francisco, CA, USA), a humanized monoclonal antibody directed against the extracellular juxtamembrane domain IV of HER2, revolutionized the treatment of HER2-positive breast cancer. This leads to decreased HER2 signaling and cell growth inhibition by several proposed mechanisms: (I) decreased ligand independent signaling; (II) increased destruction of HER2 after endocytosis; (III) immune activation; and (IV) inhibition of shedding of the extracellular domain (8). Trastuzumab has been proposed to induce antibody-dependent cellular cytotoxicity (ADCC) (9) as its IgG1 Fc heavy chain domain can bind and activate the Fc receptor of immune effector cells once trastuzumab is bound to the tumor cell (10). Consistent with this hypothesis, trastuzumab had decreased anti-tumor activity in mice with deletion of FcγR (11), whereas augmenting the response of natural killer cells enhanced antitumor activity of trastuzumab in mouse xenograft models (12). Two retrospective clinical studies correlated improved outcomes with trastuzumab with the presence of an FcR genotype that yields a stronger binding between FcR and the immune cell (13,14). In contrast, analysis from a larger cohort of patients did not find a correlation between FcR genotype and outcome with trastuzumab treatment (15). Trastuzumab also has been shown to inhibit angiogenesis, and decrease signaling through PI3K and MAPK pathways, leading to cell death (9,16,17).
In a seminal phase III study, addition of trastuzumab to chemotherapy prolonged the time to progression (TTP) and OS in patients with metastatic breast cancer (MBC) (18). In the adjuvant setting, addition of trastuzumab also resulted in improved disease free survival (DFS) and OS for patients with non-metastatic breast cancer (19-21). Indeed, trastuzumab has altered the natural history of breast cancer: emerging data indicate that patients with trastuzumab-treated HER2-positive breast cancer now have a better survival than patients with HER2-normal breast cancer (22,23).
Despite overall promising activity in HER2-positive breast cancer, only half of patients had a tumor response and 50% of patients had progression of their disease by 7.4 months, indicating that de novo and acquired resistance to HER2-targeted therapy exists (18). In general, trastuzumab-resistant tumors continue demonstrating HER2 amplification and dependence on HER2 signaling, thus the development of additional HER2-targeted agents is clinically relevant. Subsequent to the approval of trastuzumab, the oral small molecule tyrosine kinase inhibitor lapatinib became available for patients with HER2-positive MBC whose disease had progressed on prior treatment with trastuzumab, taxane and an anthracycline. In contrast to trastuzumab, lapatinib binds to the intracellular adenosine triphosphate binding domain of ErbB1 (HER1, epidermal growth factor receptor, EGFR) and HER2 (24). The regulatory approval of lapatinib was based phase III data demonstrating the median TTP for lapatinib plus capecitabine was 8.4 months compared to 4.4 months for capecitabine alone (25). Since then, clinical synergy has been demonstrated with dual HER2-directed therapy in patients whose disease had progressed on trastuzumab. A phase III study evaluating heavily pretreated patients with trastuzumab-resistant, HER2-positive MBC showed that treatment with lapatinib and trastuzumab led to prolonged OS compared to lapatinib alone (24,26).
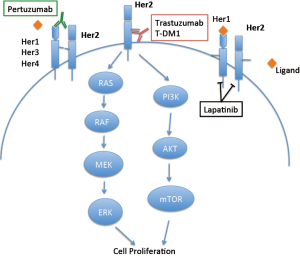
In the past 2 years, pertuzumab (Perjeta, Genentech) and ado-trastuzumab emtansine (T-DM1, Kadcyla, Genentech) have been FDA-approved for HER2-positive breast cancer. This article reviews the current evidence supporting pertuzumab and T-DM1 for HER2-positive breast cancer. Figure 1 summarizes the site of action of trastuzumab, lapatinib, pertuzumab and T-DM1 on HER2.
Pertuzumab
Mechanism of action, preclinical and phase I clinical studies
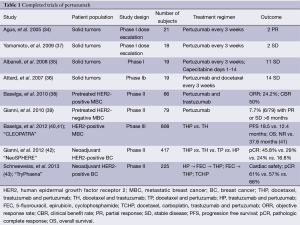
Pertuzumab is a recombinant, humanized monoclonal antibody targeting HER2. Unlike trastuzumab which binds HER2 at juxtamembrane domain IV, pertuzumab binds HER2 at the extracellular dimerization subdomain II critical for heterodimerization (Figure 1). In this way, pertuzumab more effectively blocks HER2 receptor heterodimerization with other HER family members, including EGFR, HER3, and HER4 (27,28). In preclinical models, pertuzumab inhibited tumor growth even if HER2 was not overexpressed, presumably due to inhibition of receptor heterodimerization (29-31). Pertuzumab inhibited the growth of HER2-overexpressing cell lines in vitro and potent synergy was observed with the combination of trastuzumab and pertuzumab (32,33). Tumor regression also occurred when pertuzumab was added after progression on trastuzumab alone (32). Given these promising preclinical data, a phase I clinical trial of pertuzumab in solid tumors was undertaken (34). Twenty-one patients with advanced solid tumors were included in the study and treated with 0.5 to 15 mg/kg pertuzumab every 3 weeks. A maximum tolerated dose (MTD) was not achieved, even at 15 mg/kg, the highest dose tested. Partial responses (PR) were seen in a patient with ovarian cancer and in a patient with pancreatic islet carcinoma. Neither tumor overexpressed HER2. Stable disease (SD) of 2.5 months or greater duration was seen in six patients. Three additional phase I studies have been performed, alone or in combination with chemotherapy (35-37) (Table 1).
Full table
Phase II and III studies
Given the preclinical data demonstrating synergy between trastuzumab and pertuzumab (32,33), the combination was evaluated in the metastatic setting. In a phase II single arm clinical trial, 66 patients with HER2-positive MBC who had progressed on trastuzumab were treated with trastuzumab and pertuzumab. Trastuzumab was given either as an 8 mg/kg IV loading dose followed by 6 mg/kg q3 weeks or as a 4 mg/kg loading dose followed by 2 mg/kg IV weekly, and pertuzumab was given as an 840 mg IV loading dose followed by 420 mg IV q3 weeks (38). An objective response rate (ORR) of 24.2% with a clinical benefit rate (CBR) of 50% was seen including 5 (7.6%) complete responses (CR), 11 (16.7%) PR, and 17 (25.8%) SD lasting 6 months or greater (38). Despite the promising preclinical data with pertuzumab in tumors that do not overexpress HER2 (29-31), existing clinical data published by Gianni et al. (39) suggests it should be reserved for patients with HER2-positive breast cancer. In this study (39), 79 patients with HER2-normal MBC were treated with pertuzumab given either as a 840 mg loading dose followed by 420 mg q3 weeks or as 1,050 mg q3 weeks. Only two out of 78 evaluable patients had a PR, indicating little efficacy in this population.
The promising early clinical data in HER2-positive disease led to a phase III randomized, double-blind trial, clinical evaluation of pertuzumab and trastuzumab (CLEOPATRA). In this study, 808 patients with HER2-positive MBC who had not received prior trastuzumab therapy in the metastatic setting were randomized to receive docetaxel and trastuzumab with either pertuzumab (THP) or placebo (TH). Only 11% of patients had received trastuzumab in the adjuvant or neoadjuvant setting, thus this study primarily tested the activity of dual HER2 monoclonal antibody therapy in a trastuzumab-naïve population. Median PFS was 12.4 months with placebo and 18.5 months with pertuzumab [hazard ratio (HR) 0.62 (95% CI: 0.51-0.75), P<0.0001] (40). After a median of 30 months follow-up, OS was 37.6 months [95% CI: 34·3-NE (not estimable)] for the placebo arm, and median OS had not been reached in the pertuzumab group (95% CI: 42.4-NE), (HR 0.66, 95% CI: 0.52-0.84; P=0.0008) (41). Based on these data, on June 12, 2012 the FDA approved pertuzumab in combination with trastuzumab and docetaxel for HER2-positive MBC in patients who had not received prior HER2-directed therapy or chemotherapy for metastatic disease. The European Medicines Agency (EMA) gave its approval on March 5, 2013.
Results of two landmark studies led to the September 2013 FDA approval of pertuzumab in the neoadjuvant setting for HER2-positive disease. In the phase II NeoSphere (Neoadjuvant Study of Pertuzumab and Herceptin in an Early Regimen Evaluation) study, 417 patients with HER2-positive locally advanced, inflammatory breast cancer or breast cancer with tumors >2 cm were randomized to receive four cycles of one of four regimens: pertuzumab, trastuzumab and docetaxel (THP), trastuzumab and docetaxel (TH), pertuzumab and docetaxel (TP) or pertuzumab and trastuzumab (HP). The primary endpoint was pathologic CR (pCR) rate, defined as absence of invasive cancer in the breast (ypT0/is). Treatment with THP resulted in the highest pCR rate of 45.8% (95% CI: 36.1-55.7%), compared to 29% (20.6-38.5%), 24% (1.8-33.7%) and 16.8% (10.3-25.3%) for TH, TP and HP, respectively. Rates of pCR in both the breast and axillary lymph nodes (ypT0/isypN0) were 39.3% (30-49.2%) for THP, 21.5% (14-30.5%) for TH, 17.7% (10.7-26.8%) for TP, 11.2% (5.9-18.8%) for HP. Notably, rates of pCR in the hormone receptor positive (ER+) group were lower compared to those that were hormone receptor negative (ER–): 22% vs. 54.4% (THP), 12% vs. 29.8% (TH), 8.7% vs. 26% (TP), and 2% vs. 20% (HP) for ER-positive vs. ER–, respectively (42).
Similarly, TryPhaena (ToleRabilitY of Pertuzumab, Herceptin and AnthracyclinEs in NeoAdjuvant breast cancer) demonstrated favorable results with the addition of pertuzumab to trastuzumab and chemotherapy in the neoadjuvant setting (43). In this phase II study, 225 patients were enrolled onto one of three arms: (I) Arm A—three cycles of pertuzumab, trastuzumab with concurrent 5-fluorouracil, epirubicin, cyclophosphamide (FEC) followed by three cycles of docetaxel, pertuzumab and trastuzumab; (II) Arm B—three cycles of FEC followed by three cycles of docetaxel, pertuzumab and trastuzumab; and (III) Arm C—six cycles of docetaxel, carboplatin, pertuzumab and trastuzumab (TCHP) chemotherapy. Though the primary outcome of the study was cardiac safety, the secondary outcome of pCR in the breast (ypT0/is) or breast and axilla (ypT0/isyN0) was impressive in all three arms. Breast only pCR rates were 61.6% (56.2% ypT0/isyPN0, Arm A), 57.3% (54.7% ypT0/isypN0, Arm B), and 66.2% (63.6%, ypT0/isypN0, Arm C). The FDA approved three neoadjuvant regimens for HER2-positive breast cancer: FEC ×3 followed by THP ×3, TCHP ×6, and THP ×4 followed by surgery and FEC.
Ongoing clinical studies of pertuzumab
Several studies with pertuzumab are ongoing. In the frontline metastatic setting, PHEREXA (NCT01026142) will evaluate whether pertuzumab and trastuzumab with capecitabine will improve PFS compared to trastuzumab and capecitabine and PERUSE will evaluate the combination of pertuzumab, trastuzumab and taxane in 1,500 patients. PERTAIN (NCT01491737) is a phase II study randomizing 250 patients with ER+ and HER2-positive MBC to either trastuzumab, aromatase inhibitor +/– pertuzumab or taxane, trastuzumab +/– pertuzumab followed by trastuzumab, aromatase inhibitor +/– pertuzumab. Finally, VELVET is evaluating pertuzumab, trastuzumab and vinorelbine in a single arm phase II frontline study in patients with HER2-positive MBC.
Whether the addition of pertuzumab to a trastuzumab-based chemotherapy regimen in the adjuvant setting will improve outcomes is currently being evaluated in a 4,880-patient phase III clinical trial (APHINITY, NCT01358877) that completed accrual in August 2013. In addition, a phase II neoadjuvant study (KRISTINE, NCT02131064) will evaluate six cycles of neoadjuvant TCHP followed by trastuzumab and pertuzumab post-operatively (Arm A) compared to six cycles of neoadjuvant T-DM1 and pertuzumab followed by T-DM1 and pertuzumab post-operatively (Arm B) to complete one year of adjuvant therapy.
Adverse events (AE) for pertuzumab
In the phase I study, the most common side effects of pertuzumab alone were grade 1 or 2 rash or diarrhea (34). These anti-EGFR toxicities are consistent with its mechanism of action as an inhibitor of HER2 heterodimerization with EGFR. The most frequent AE of pertuzumab in combination with trastuzumab were grade 1 or 2 diarrhea (64%), fatigue (33%) and nausea (27%) (40). The most common side effects of docetaxel, trastuzumab and pertuzumab were diarrhea, alopecia, neutropenia, nausea, fatigue, and rash. Among these, only diarrhea and rash were increased more than 5% compared docetaxel and trastuzumab. A greater than 5% increase in incidence of mucosal inflammation, febrile neutropenia and dry skin was also seen over the control arm (40). About half of the women in TryPhaena who received docetaxel developed neutropenia, however, fewer than 10% developed febrile neutropenia (42). An increased incidence of febrile neutropenia, rash, mucosal inflammation was seen, in addition to diarrhea. When docetaxel was discontinued, most side effects, except for diarrhea, pruritis and rash, resolved (40).
Though an early study in eleven patients raised concerns for cardiac side effects of pertuzumab (44), adding pertuzumab to trastuzumab and chemotherapy does not increase the risk of cardiotoxicity (38,41,43,45). In the phase II study of pertuzumab and trastuzumab, three of 66 patients had a decrease in LVEF >10% from baseline and an absolute LVEF <50% during the course of the study. All were asymptomatic (38). Rates of any grade LV dysfunction were 4.4% for THP and 8.3% for TH. Grade 3 LV systolic dysfunction was seen in 1.2% of patients treated with pertuzumab vs. 2.8% in the control group (40). In CLEOPATRA, a 9% incidence of LVEF decline and 1% symptomatic LV dysfunction was seen with THP, compared to 5% and 2% for TH, respectively, and the difference was not statistically significant. Furthermore, in the neoadjuvant TryPhaena in which the primary endpoint was cardiac safety, only two patients, both in the FEC→THP arm, experienced symptomatic left ventricular systolic dysfunction. Eleven patients of 225 had decreases in LVEF
Taken together, the published data indicate that adding pertuzumab to chemotherapy and trastuzumab improves PFS and OS and does so with manageable side effects. To this end, the 2014 National Comprehensive Cancer Network (NCCN) and American Society of Clinical Oncology (ASCO) Clinical Practice guidelines both recommended pertuzumab and trastuzumab with taxane chemotherapy as the preferred option for first-line therapy of HER2-positive MBC (49).
T-DM1
Mechanism of action and preclinical data
T-DM1 is another HER2 directed antibody therapy recently approved for treatment of HER2-positive breast cancer. T-DM1 is a novel antibody-drug conjugate (ADC) whereby trastuzumab is stably linked to the microtubule-inhibitor, DM-1. DM-1 is a derivative of maytansine, a potent tubulin-binder originally identified from plant species including Rhamnaceae and Euphorbiaceae, as well as some mosses (50). Maytansine inhibits microtubule polymerization and promotes depolymerization with a potency 100-fold greater than vinca alkaloids, however, dose-limiting neuropathy, diarrhea and fatigue prevented its clinical development (51,52). DM-1 is a maytansinoid with a thio-containing moiety that allows for linkage of DM-1 to antibodies via disulfide bonds. Given the potency of maytansine, the clinical activity of microtubule inhibitors such as taxanes for breast cancer, and the selectivity of HER2 as a target of antibody-directed therapies, a variety of reducible and non-reducible thioether linkers between trastuzumab and DM-1 were evaluated to identify an ADC for trastuzumab. Among these, T-DM1 was the most potent and least toxic of a series of trastuzumab-linked DM-1 molecules tested (51,53,54). T-DM1 is synthesized by covalently binding the linker molecule SMCC [N-succinimidyl-4-(maleimidomethyl) cyclohexanecarboxylate] to the lysine residues on trastuzumab and then reacting the maleimide moiety of SMCC with the thioether group of DM-1. T-DM1 contains an average of 3-3.5 DM1 molecules for each trastuzumab antibody (53).
T-DM1 exerts its anti-tumor effect via a variety of mechanisms. After binding of T-DM1 to HER2 on the breast cancer cell, the HER2-T-DM1 complex is endocytosed and T-DM1 is proteolytically degraded within the cell lysosome, allowing release of Lys-SCC-DM-1 (55). Lys-SCC-DM-1, via its inhibition of microtubules, causes cell cycle arrest and cell death (53). As it cannot cross the cell membrane, cytotoxic effects only occur in the cell in which it is internalized without affecting neighboring cells (56). Thus, T-DM1 functions as a targeted cytotoxic agent. T-DM1 binds HER2 with the same binding affinity as trastuzumab (57). It both inhibits HER2 cell signaling and targets cells for ADCC, consistent with the mechanism of action of trastuzumab itself (53,57).
Phase I studies of single agent T-DM1
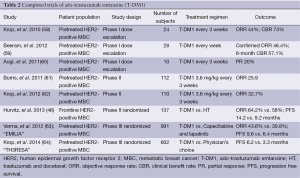
Table 2 summarizes the completed clinical trials of T-DM1. The safety and tolerability of T-DM1 was first evaluated in a multi-center, open label phase I dose escalation study. T-DM1 was given either weekly (n=28) (59) or every 3 weeks (n=24) (58) to patients with HER2-positive MBC that had progressed on trastuzumab. All patients had received at least one microtubule inhibitor and four patients had received more than two. Patients were heavily pretreated as they had received a median of 4 prior lines of therapy (range, 1-8). In the q3 week arm, T-DM1 was evaluated at doses ranging from 0.3 to 4.8 mg/kg IV every three weeks (58). The dose-limiting toxicity (DLT) of transient thrombocytopenia occurred at 4.8 mg/kg, and the MTD/recommended phase 2 dose was 3.6 mg/kg IV every 3 weeks. Of the 24 patients treated, six PR were observed. Among the nine patients treated at the MTD who had measurable disease, four (44%) patients had a confirmed PR, with a clinical benefit rate of 73%.
Full table
The initial weekly dose was 1.2 mg/kg weekly and the MTD was 2.9 mg/kg weekly. The DLT was thrombocytopenia. Thirteen of 28 patients (46.4%) had confirmed objective PR with a median duration of response of 18.6 months and a 57.1% 6-month clinical benefit rate (59). This dosing schedule appeared to be relatively well tolerated, though 68% of patients reported AEs grade 3 or worse.
Aogi et al. (60) described results of a third phase I study in 10 Japanese patients. T-DM1 was administered q3 weeks at 1.8, 2.4 and 3.6 mg/kg. One grade 3 transaminitis was reported and the MTD was identified as 3.6 mg/kg. Two PR were observed.
Phase II studies of single agent T-DM1
Given the promising activity seen in phase I studies, several phase II studies have been completed with the 3.6 mg/kg q3-week dosing schedule. TDM4258g was a single-arm proof-of-concept study that enrolled 112 patients with HER2-positive MBC who progressed on HER2-directed therapy (61). Patients had received a median of eight prior therapies; all had received prior trastuzumab and almost two-thirds (66/112) had received prior lapatinib. By independent review, the ORR was 25.9% (95% CI: 18.4-34.4%). Of 75 patients who had previously discontinued trastuzumab due to progression, 21 achieved objective responses (ORR 28.0%, 95% CI: 18.2-38.9%). Of the 66 patients who previously had received lapatinib, the ORR was 24.2% (95% CI: 14.5-36.0%). The median PFS was 4.6 months (95% CI: 3.9-8.6 months) (61).
Krop et al. (62) published the results of TDM4374g, a confirmatory phase II study of T-DM1 in 110 patients who previously received chemotherapy and two HER2-directed therapies including lapatinib and trastuzumab. The ORR was 32.7% (95% CI: 24.1-42.1%) and median PFS 7.2 months.
In the frontline setting, T-DM1 was compared head-to-head with trastuzumab plus docetaxel (HT) in a randomized phase II trial for the treatment of HER2-positive locally advanced or MBC (TDM4450g) (65). A total of 137 subjects were enrolled. Patients were eligible to enroll if they had not received chemotherapy for metastatic disease and if they were ≥6 months from prior chemotherapy in the adjuvant setting. Sixty-seven patients were treated with T-DM1, compared to 70 patients treated with HT. Toxicity and investigator assessed PFS were the primary endpoints. The median PFS was 14.2 months for T-DM1 vs. 9.2 months for HT (HR 0.59; 95% CI: 0.36 to 0.97; P=0.035). There were three CRs in the HT arm and seven CRs in the T-DM1 arm (P=0.453). For patients who received T-DM1, the ORR was 64.2% (95% CI: 51.8% to 74.8%) compared to 58.0% (95% CI: 45.5% to 69.2%) for HT. OS was similar between the two arms, although at the time of reporting, only 13 deaths had occurred. Compared to HT, fewer grade 3/4 AEs were seen in the T-DM1 arm (46.4% vs. 90.9% for TDM-1 and HT, respectively). Overall, T-DM1 treatment resulted in fewer treatment discontinuations (7.2%) compared to for HT (34.8%) and fewer serious AEs (20.3% vs. 25.8%).
Phase III studies
The phase III randomized EMILIA trial (TDM4370) unequivocally demonstrated the efficacy of T-DM1 in patients with HER2-positive, trastuzumab-pretreated MBC (63). A total of 991 subjects with HER2-positive advanced breast cancer, previously treated with taxane and trastuzumab were randomized to receive TDM-1 or lapatinib plus capecitabine. Primary endpoints were independently assessed PFS, OS, and safety. A statistically significant improvement in ORR was seen with T-DM1 compared with lapatinib and capecitabine (43.6% vs. 30.8%, P<0.001). Median PFS was 9.6 months for T-DM1 vs. 6.4 months with lapatinib and capecitabine (HR 0.65; 95% CI: 0.55 to 0.77; P<0.001), and median OS at the second interim analysis was 30.9 vs. 25.1 months (HR 0.68; 95% CI: 0.55 to 0.85; P<0.001). Fewer grade 3 or greater toxicities were seen with T-DM1 compared to lapatinib and capecitabine, with rates of 41% and 57%, respectively. Thrombocytopenia and elevated transaminases were more common with T-DM1, while diarrhea, nausea, vomiting, palmar-plantar dysesthesia were more common in the lapatinib and capecitabine arm. Based on this seminal result, both the FDA and EMA have licensed T-DM1 as monotherapy for HER2-positive MBC in patients who had previously received taxane and trastuzumab-based therapy (66).
TH3RESA was a randomized, open label trial evaluating T-DM1 vs. treatment of physician’s choice in patients who had previously received two or more HER2 directed therapies, including trastuzumab and lapatinib as well as taxane chemotherapy (64). A total of 602 patients were enrolled: 404 received T-DM1 and 198 patients received therapy per physician’s choice. At the time of reporting, 44 patients had crossed over to the T-DM1 arm. For the T-DM1 arm, median PFS was 6.2 months, compared to 3.3 months for TPC (stratified HR 0.528, 0.422-0.661, P<0.001). Interim OS data also demonstrated a trend toward improvement in the T-DM1 arm (HR 0.552, 95% CI: 0.369-0.826, P=0.0034). T-DM1 treatment resulted in fewer grade 3 or greater AE compared to TPC: 32% vs. 43%, respectively. Grade 3 thrombocytopenia was the only AE more frequently seen with T-DM1 and was seen in 5% of patients treated with T-DM1, compared with 2% in the control arm. Grade 3 neutropenia, diarrhea and febrile neutropenia were all more common for TPC than for T-DM1 arm.
Ongoing studies
Several additional phase II and III studies with T-DM1 are ongoing. MARIANNE (NCT01120184) is a three-arm phase III study evaluating the combination of pertuzumab and T-DM1 in HER2-positive first-line locally advanced or MBC. Patients will be randomized to receive T-DM1 alone, T-DM1 with pertuzumab, or trastuzumab plus taxane (docetaxel or paclitaxel). Clinical trials in non-metastatic HER2-positive breast cancer are also underway. NCT01196052 (TDM4874g) is a single arm, phase II study of T-DM1 in the adjuvant and neoadjuvant setting. The primary endpoint is safety. In the phase II randomized study, ATEMPT (NCT01853748), T-DM1 will be compared to paclitaxel and trastuzumab in patients with resected stage I disease. And two neoadjuvant studies are ongoing with the primary endpoint of pCR rate: ADAPT is evaluating T-DM1 vs. trastuzumab (NCT01745965) and I-SPY2 (NCT01042379) is comparing T-DM1 and pertuzumab with standard therapy. Moreover, as previously described, KRISTINE (NCT02131064) compares six 3-week cycles of neoadjuvant T-DM1 and pertuzumab (Arm A) with TCHP (Arm B), followed by T-DM1 and pertuzumab (Arm A) or trastuzumab and pertuzumab (Arm B) after surgery.
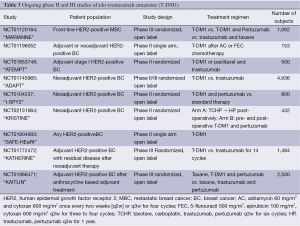
Cardiac safety will be the primary endpoint for NCT010904903 (“SAFE”-HEaRt), an open-label, phase II study of T-DM1 in HER2-positive MBC. KATHERINE (NCT01772472) is a randomized, multicenter, open label phase III study evaluating the efficacy and safety of T-DM1 vs. trastuzumab as adjuvant therapy for patients with HER2-positive breast cancer with residual disease in the breast or axillary lymph nodes following neoadjuvant therapy. Patients receive T-DM1 or trastuzumab q3 weeks for 14 cycles. Radiotherapy and hormonal therapy may also be given as indicated. The randomized, open label study KAITLIN (NCT01966471) will evaluate the safety and efficacy of taxane-based adjuvant therapy with either T-DM1 and pertuzumab or trastuzumab and pertuzumab in patients who have undergone surgery and adjuvant anthracycline-based chemotherapy. Table 3 summarizes the ongoing phase III trials of T-DM1.
Full table
Notable adverse events with T-DM1
The most frequent AEs for T-DM1 are transaminitis and thrombocytopenia. At the MTD the most common AEs were grade 1 or 2 thrombocytopenia, transaminitis, fatigue, nausea and anemia (58-60). Aogi reported 10% of AEs were grade 3 and 0.4% were grade 4 (60). Phase II studies confirmed the side effect profile (61,62). Notably, despite the fact that DM-1 is a cytotoxic microtubule inhibitor, very little neuropathy has been observed and alopecia is uncommon. Serious AEs were reported in 23% of patients, 42% of these grade 3 or 4, including thrombocytopenia (5.4%), back pain (3.6%), fatigue (2.7%), and transaminitis (2.7%) (62).
Though the most frequent grade 3 or 4 toxicity of T-DM1 was thrombocytopenia (58,59,61-63,65), hemorrhagic events are rare. Furthermore, while thrombocytopenia at times required dose reduction, no patients have discontinued study due to hemorrhage. Platelet counts usually nadir around day 8 and recover by day 15.
The mechanism of thrombocytopenia is currently under investigation. In murine models, though murine megakaryocytes do not express HER2, T-DM1 is endocytosed into megakaryocytes and inhibits microtubule assembly (67,68). This effect was confirmed in platelets from healthy human donors and may be mediated by surface FcϒRIIb (69). T-DM1 does not affect platelet function as platelet aggregation and activation of healthy human donor platelets were not altered by treatment of washed platelets or platelet rich plasma with T-DM1 (69). Instead, thrombocytopenia may be related to altered platelet production by megakaryocytes in the bone marrow (69). In vitro and population pharmacokinetic modeling studies developed with data from TDM3569g and TDM4258g and validated with data from TDM4374g concluded that the thrombocytopenia may be due to depletion of platelet pools (70).
T-DM1 can cause transaminitis, but grade 3 transaminitis was generally <10% across all studies. Most instances of transaminitis resolve with dose reduction. One death has been attributed to abnormal liver function. This patient had underlying steatohepatitis and multiple other medical issues (62).
Cardiac AE are also very rare with T-DM1. In TDM4258g, no grade 3 decline in LVEF or symptomatic congestive heart failure was observed and only two patients had LVEF decrease to 40-45%. There were no treatment discontinuations due to cardiotoxicity (61). Similarly, there were no incidences of decreased EF >25% and no patients had LVEF <45 by the end of study in TDM4374g (62). Of note, many of the patients enrolled on clinical studies of T-DM1 had received prior trastuzumab; therefore one possibility for the relatively low incidence of cardiac AEs is selection bias: patients at risk from cardiac dysfunction due to HER2-targeted therapies would already have been excluded from entering these studies. The ongoing frontline study comparing T-DM1 and trastuzumab (MARIANNE) in HER2-positive MBC will clarify this issue.
Biomarkers and predictors of response to T-DM1
Exploratory data indicate that tumor HER2-mRNA levels may be predictive of likelihood of response and duration of response in patients treated with T-DM1 (61,62,71). In the proof-of-concept phase II study (61), 74 of 95 evaluable specimens were independently confirmed as being HER2-positive by immunohistochemistry or FISH. ORR was 33.8% among those with HER2-positive tumors, compared to 4.8% in HER2 normal tumors. Median PFS was 8.2 vs. 2.6 months for HER2-positive vs. HER2-normal subjects, respectively. Among HER2-positive tumors, those with HER2 mRNA levels above the median had an ORR of 36% (95% CI: 18.5-56.9%) and impressively, median PFS (95% CI: 4.4 months to NE) was not reached. Those with HER2 mRNA levels below the median had ORR 28% (95% CI: 12.1-47.5%) and median PFS 4.2 months (95% CI: 2.7-6.8 months). Similar data were reported by LoRusso et al. (72) which included retrospective analysis of TDM4374g and by Perez et al. (71) who analyzed data from the frontline, randomized phase II study (65). While patients with HER2 mRNA levels at or above the median had an ORR of 72.4% (29/67) and the median PFS was not reached, those with mRNA levels below the median had a 53.8% (26/67) ORR and 10.6 month median PFS (71). Notably, median PFS was 8.8 months in those with mRNA levels at or above the median vs. 9.8 months for those below the median for the HT arm (71).
PIK3CA activating mutations have been associated with decreased response to HER2-targeted therapy (73). However, in a subgroup analysis of EMILIA, Baselga et al. demonstrated that while patients with PIK3CA mutations who received capecitabine and lapatinib had shorter median PFS and OS, PIK3CA mutation status did not affect outcomes in the T-DM1 group (74).
Combination anti-HER2 antibody therapy
As combining pertuzumab and trastuzumab is synergistic (32,33,40), combining pertuzumab and T-DM1 is an active area of research. Phillips et al. evaluated this combination preclinically and clinically in a phase Ib/II study (75). Combining T-DM1 and pertuzumab led to synergistic cell growth inhibition and induction of apoptosis in vitro, and synergy was also seen in murine xenograft models. In some cell lines, addition of neuregulin, the HER3 ligand, decreased the inhibitory effect of T-DM1, but the effect of neuregulin could be counteracted by addition of pertuzumab. In their phase Ib/II study evaluating the safety and tolerability of TDM-1 with pertuzumab, the ORR was 44% for the phase Ib portion. For the phase II portion of the study, Miller et al. reported that nine PR were seen among 23 evaluable patients (76). A total enrollment of 60 patients is planned and final results of the phase II portion of the study are eagerly awaited. TDM4652g is a phase Ib study evaluating the combination of T-DM1 (weekly or every three weeks), weekly paclitaxel and pertuzumab every three weeks in patients with trastuzumab pre-treated HER2-positive MBC. The MTD was 2.4 mg/kg T-DM1 weekly, paclitaxel 80 mg/m2 weekly and pertuzumab 840 mg loading, with 420 mg q3 weeks in the weekly T-DM1 arm (77); additional results are forthcoming. TDM1 and docetaxel with and without pertuzumab is also being evaluated in the neoadjuvant setting (78). A second neoadjuvant phase II study KRISTINE is ongoing to evaluate TDM-1 and pertuzumab pre- and post-operatively compared to TCHP followed by trastuzumab and pertuzumab. Finally, the phase III study MARIANNE will formally evaluate the efficacy of pertuzumab and T-DM1 compared to T-DM1 alone or trastuzumab with taxane in the frontline metastatic or locally advanced setting (Table 3).
Conclusions and future directions
Treatment of HER2-positive breast cancer improved significantly with the incorporation of HER2-directed therapies such as trastuzumab and lapatinib. Further advances have been made with the addition of pertuzumab and T-DM1, which both are relatively well-tolerated and have activity in HER2-positive disease refractory to trastuzumab and/or lapatinib. The improved outcomes seen with the use of trastuzumab, pertuzumab and T-DM1 for HER2-positive disease underscores the potential of HER2-directed antibody and ADCs for HER2-positive breast cancer. To this end, several novel antibodies and ADCs are in early clinical development including MM302, an antibody linked to pegylated liposomal doxorubicin (NCT01304797) and MM111, an antibody fusion protein targeting the HER2/HER3 heterodimer (79). Active clinical research and development of novel therapies and combinations will hopefully result in continued improvements in outcomes for patients with HER2-positive breast cancer.
Acknowledgements
Dr. Hurvitz has served as institutional PI on Genentech and Roche-sponsored clinical trials and has received grant funding (paid to the institution). Travel was paid by Genentech for speaking at an international conference.
Disclosure: The authors declare no conflict of interest.
References
- Slamon DJ, Godolphin W, Jones LA, et al. Studies of the HER-2/neu proto-oncogene in human breast and ovarian cancer. Science 1989;244:707-12. [PubMed]
- Wolff AC, Hammond ME, Hicks DG, et al. Recommendations for human epidermal growth factor receptor 2 testing in breast cancer: American Society of Clinical Oncology/College of American Pathologists clinical practice guideline update. J Clin Oncol 2013;31:3997-4013. [PubMed]
- Yarden Y, Sliwkowski MX. Untangling the ErbB signalling network. Nat Rev Mol Cell Biol 2001;2:127-37. [PubMed]
- Slamon DJ, Clark GM, Wong SG, et al. Human breast cancer: correlation of relapse and survival with amplification of the HER-2/neu oncogene. Science 1987;235:177-82. [PubMed]
- Perou CM, Sørlie T, Eisen MB, et al. Molecular portraits of human breast tumours. Nature 2000;406:747-52. [PubMed]
- Sørlie T, Perou CM, Tibshirani R, et al. Gene expression patterns of breast carcinomas distinguish tumor subclasses with clinical implications. Proc Natl Acad Sci U S A 2001;98:10869-74. [PubMed]
- Venter DJ, Tuzi NL, Kumar S, et al. Overexpression of the c-erbB-2 oncoprotein in human breast carcinomas: immunohistological assessment correlates with gene amplification. Lancet 1987;2:69-72. [PubMed]
- Hudis CA. Trastuzumab--mechanism of action and use in clinical practice. N Engl J Med 2007;357:39-51. [PubMed]
- Yamauchi C, Fujii S, Kimura T, et al. E-cadherin expression on human carcinoma cell affects trastuzumab-mediated antibody-dependent cellular cytotoxicity through killer cell lectin-like receptor G1 on natural killer cells. Int J Cancer 2011;128:2125-37. [PubMed]
- Spector NL, Blackwell KL. Understanding the mechanisms behind trastuzumab therapy for human epidermal growth factor receptor 2-positive breast cancer. J Clin Oncol 2009;27:5838-47. [PubMed]
- Clynes RA, Towers TL, Presta LG, et al. Inhibitory Fc receptors modulate in vivo cytotoxicity against tumor targets. Nat Med 2000;6:443-6. [PubMed]
- Kohrt HE, Houot R, Weiskopf K, et al. Stimulation of natural killer cells with a CD137-specific antibody enhances trastuzumab efficacy in xenotransplant models of breast cancer. J Clin Invest 2012;122:1066-75. [PubMed]
- Musolino A, Naldi N, Bortesi B, et al. Immunoglobulin G fragment C receptor polymorphisms and clinical efficacy of trastuzumab-based therapy in patients with HER-2/neu-positive metastatic breast cancer. J Clin Oncol 2008;26:1789-96. [PubMed]
- Tamura K, Shimizu C, Koizumi F, et al. Correlation of FcγR IIa-H131R and IIIa-V158F polymorphisms and clinical outcome of trastuzumab in both neoadjuvant and metastatic setting in patients with HER-2 positive breast cancer. J Clin Oncol 2009;27:abstr 1100.
- Hurvitz S, Betting D, Stern H, et al. Analysis of Fc{gamma} Receptor IIA & IIIA Polymorphisms: Correlation with Outcome in Trastuzumab-Treated HER2/Neu Amplified Early and Metastatic Breast Cancer Patients. Cancer Res 2009;69:Abstract nr 64.
- Miller TW, Rexer BN, Garrett JT, et al. Mutations in the phosphatidylinositol 3-kinase pathway: role in tumor progression and therapeutic implications in breast cancer. Breast Cancer Res 2011;13:224. [PubMed]
- Nahta R. Pharmacological strategies to overcome HER2 cross-talk and Trastuzumab resistance. Curr Med Chem 2012;19:1065-75. [PubMed]
- Slamon DJ, Leyland-Jones B, Shak S, et al. Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER2. N Engl J Med 2001;344:783-92. [PubMed]
- Piccart-Gebhart MJ, Procter M, Leyland-Jones B, et al. Trastuzumab after adjuvant chemotherapy in HER2-positive breast cancer. N Engl J Med 2005;353:1659-72. [PubMed]
- Romond EH, Perez EA, Bryant J, et al. Trastuzumab plus adjuvant chemotherapy for operable HER2-positive breast cancer. N Engl J Med 2005;353:1673-84. [PubMed]
- Slamon D, Eiermann W, Robert N, et al. Adjuvant trastuzumab in HER2-positive breast cancer. N Engl J Med 2011;365:1273-83. [PubMed]
- Dawood S, Broglio K, Esteva FJ, et al. Defining prognosis for women with breast cancer and CNS metastases by HER2 status. Ann Oncol 2008;19:1242-8. [PubMed]
- Musolino A, Ciccolallo L, Panebianco M, et al. Multifactorial central nervous system recurrence susceptibility in patients with HER2-positive breast cancer: epidemiological and clinical data from a population-based cancer registry study. Cancer 2011;117:1837-46. [PubMed]
- Blackwell KL, Burstein HJ, Storniolo AM, et al. Randomized study of Lapatinib alone or in combination with trastuzumab in women with ErbB2-positive, trastuzumab-refractory metastatic breast cancer. J Clin Oncol 2010;28:1124-30. [PubMed]
- Geyer CE, Forster J, Lindquist D, et al. Lapatinib plus capecitabine for HER2-positive advanced breast cancer. N Engl J Med 2006;355:2733-43. [PubMed]
- Blackwell KL, Burstein HJ, Storniolo AM, et al. Overall survival benefit with lapatinib in combination with trastuzumab for patients with human epidermal growth factor receptor 2-positive metastatic breast cancer: final results from the EGF104900 Study. J Clin Oncol 2012;30:2585-92. [PubMed]
- Agus DB, Akita RW, Fox WD, et al. Targeting ligand-activated ErbB2 signaling inhibits breast and prostate tumor growth. Cancer Cell 2002;2:127-37. [PubMed]
- Badache A, Hynes NE. A new therapeutic antibody masks ErbB2 to its partners. Cancer Cell 2004;5:299-301. [PubMed]
- Agus DB, Akita RW, Fox WD, et al. A potential role for activated HER-2 in prostate cancer. Semin Oncol 2000;27:76-83; discussion 92-100. [PubMed]
- Agus DB, Akita RW, Fox WD, et al. Targeting ligand-activated ErbB2 signaling inhibits breast and prostate tumor growth. Cancer Cell 2002;2:127-37. [PubMed]
- Malik MA, Totpal K, Balter I, et al. Dose-response studies of recombinant humanized monoclonal antibody 2C4 in tumor xenograft models [abstract 773]. Proc AACR 2003;44:S176.
- Scheuer W, Friess T, Burtscher H, et al. Strongly enhanced antitumor activity of trastuzumab and pertuzumab combination treatment on HER2-positive human xenograft tumor models. Cancer Res 2009;69:9330-6. [PubMed]
- Nahta R, Hung MC, Esteva FJ. The HER-2-targeting antibodies trastuzumab and pertuzumab synergistically inhibit the survival of breast cancer cells. Cancer Res 2004;64:2343-6. [PubMed]
- Agus DB, Gordon MS, Taylor C, et al. Phase I clinical study of pertuzumab, a novel HER dimerization inhibitor, in patients with advanced cancer. J Clin Oncol 2005;23:2534-43. [PubMed]
- Albanell J, Montagut C, Jones ET, et al. A phase I study of the safety and pharmacokinetics of the combination of pertuzumab (rhuMab 2C4) and capecitabine in patients with advanced solid tumors. Clin Cancer Res 2008;14:2726-31. [PubMed]
- Attard G, Kitzen J, Blagden SP, et al. A phase Ib study of pertuzumab, a recombinant humanised antibody to HER2, and docetaxel in patients with advanced solid tumours. Br J Cancer 2007;97:1338-43. [PubMed]
- Yamamoto N, Yamada Y, Fujiwara Y, et al. Phase I and pharmacokinetic study of HER2-targeted rhuMAb 2C4 (Pertuzumab, RO4368451) in Japanese patients with solid tumors. Jpn J Clin Oncol 2009;39:260-6. [PubMed]
- Baselga J, Gelmon KA, Verma S, et al. Phase II trial of pertuzumab and trastuzumab in patients with human epidermal growth factor receptor 2-positive metastatic breast cancer that progressed during prior trastuzumab therapy. J Clin Oncol 2010;28:1138-44. [PubMed]
- Gianni L, Lladó A, Bianchi G, et al. Open-label, phase II, multicenter, randomized study of the efficacy and safety of two dose levels of Pertuzumab, a human epidermal growth factor receptor 2 dimerization inhibitor, in patients with human epidermal growth factor receptor 2-negative metastatic breast cancer. J Clin Oncol 2010;28:1131-7. [PubMed]
- Baselga J, Cortés J, Kim SB, et al. Pertuzumab plus trastuzumab plus docetaxel for metastatic breast cancer. N Engl J Med 2012;366:109-19. [PubMed]
- Swain SM, Kim SB, Cortés J, et al. Pertuzumab, trastuzumab, and docetaxel for HER2-positive metastatic breast cancer (CLEOPATRA study): overall survival results from a randomised, double-blind, placebo-controlled, phase 3 study. Lancet Oncol 2013;14:461-71. [PubMed]
- Gianni L, Pienkowski T, Im YH, et al. Efficacy and safety of neoadjuvant pertuzumab and trastuzumab in women with locally advanced, inflammatory, or early HER2-positive breast cancer (NeoSphere): a randomised multicentre, open-label, phase 2 trial. Lancet Oncol 2012;13:25-32. [PubMed]
- Schneeweiss A, Chia S, Hickish T, et al. Pertuzumab plus trastuzumab in combination with standard neoadjuvant anthracycline-containing and anthracycline-free chemotherapy regimens in patients with HER2-positive early breast cancer: a randomized phase II cardiac safety study (TRYPHAENA). Ann Oncol 2013;24:2278-84. [PubMed]
- Portera CC, Walshe JM, Rosing DR, et al. Cardiac toxicity and efficacy of trastuzumab combined with pertuzumab in patients with [corrected] human epidermal growth factor receptor 2-positive metastatic breast cancer. Clin Cancer Res 2008;14:2710-6. [PubMed]
- Swain SM, Ewer MS, Cortés J, et al. Cardiac tolerability of pertuzumab plus trastuzumab plus docetaxel in patients with HER2-positive metastatic breast cancer in CLEOPATRA: a randomized, double-blind, placebo-controlled phase III study. Oncologist 2013;18:257-64. [PubMed]
- Hurvitz SA, Miller JM, Dichmann R, et al. Abstract S1-02: Final analysis of a phase II 3-arm randomized trial of neoadjuvant trastuzumab or lapatinib or the combination of trastuzumab and lapatinib, followed by six cycles of docetaxel and carboplatin with trastuzumab and/or lapatinib in patients with HER2+ breast cancer (TRIO-US B07). Cancer Res 2013;73:Abstract nr S1-02.
- Swain SM, Im YH, Im SA, et al. Safety profile of Pertuzumab with Trastuzumab and Docetaxel in patients from Asia with human epidermal growth factor receptor 2-positive metastatic breast cancer: results from the phase III trial CLEOPATRA. Oncologist 2014;19:693-701. [PubMed]
- Miles D, Baselga J, Amadori D, et al. Treatment of older patients with HER2-positive metastatic breast cancer with pertuzumab, trastuzumab, and docetaxel: subgroup analyses from a randomized, double-blind, placebo-controlled phase III trial (CLEOPATRA). Breast Cancer Res Treat 2013;142:89-99. [PubMed]
- Giordano SH, Temin S, Kirshner JJ, et al. Systemic therapy for patients with advanced human epidermal growth factor receptor 2-positive breast cancer: American Society of Clinical Oncology clinical practice guideline. J Clin Oncol 2014;32:2078-99. [PubMed]
- Yu TW, Bai L, Clade D, et al. The biosynthetic gene cluster of the maytansinoid antitumor agent ansamitocin from Actinosynnema pretiosum. Proc Natl Acad Sci U S A 2002;99:7968-73. [PubMed]
- Cassady JM, Chan KK, Floss HG, et al. Recent developments in the maytansinoid antitumor agents. Chem Pharm Bull (Tokyo) 2004;52:1-26. [PubMed]
- Remillard S, Rebhun LI, Howie GA, et al. Antimitotic activity of the potent tumor inhibitor maytansine. Science 1975;189:1002-5. [PubMed]
- Lewis Phillips GD, Li G, Dugger DL, et al. Targeting HER2-positive breast cancer with trastuzumab-DM1, an antibody-cytotoxic drug conjugate. Cancer Res 2008;68:9280-90. [PubMed]
- Widdison WC, Wilhelm SD, Cavanagh EE, et al. Semisynthetic maytansine analogues for the targeted treatment of cancer. J Med Chem 2006;49:4392-408. [PubMed]
- Erickson HK, Park PU, Widdison WC, et al. Antibody-maytansinoid conjugates are activated in targeted cancer cells by lysosomal degradation and linker-dependent intracellular processing. Cancer Res 2006;66:4426-33. [PubMed]
- Xie H, Audette C, Hoffee M, et al. Pharmacokinetics and biodistribution of the antitumor immunoconjugate, cantuzumab mertansine (huC242-DM1), and its two components in mice. J Pharmacol Exp Ther 2004;308:1073-82. [PubMed]
- Junttila TT, Li G, Parsons K, et al. Trastuzumab-DM1 (T-DM1) retains all the mechanisms of action of trastuzumab and efficiently inhibits growth of lapatinib insensitive breast cancer. Breast Cancer Res Treat 2011;128:347-56. [PubMed]
- Krop IE, Beeram M, Modi S, et al. Phase I study of trastuzumab-DM1, an HER2 antibody-drug conjugate, given every 3 weeks to patients with HER2-positive metastatic breast cancer. J Clin Oncol 2010;28:2698-704. [PubMed]
- Beeram M, Krop IE, Burris HA, et al. A phase 1 study of weekly dosing of trastuzumab emtansine (T-DM1) in patients with advanced human epidermal growth factor 2-positive breast cancer. Cancer 2012;118:5733-40. [PubMed]
- Aogi K, Ando M, Iwata H, et al. P1-12-19: Phase I Study of Single Agent Trastuzumab Emtansine in Japanese Patients with Human Epidermal Growth Factor Receptor2 (HER2)-Positive Metastatic Breast Cancer (JO22591). Cancer Res 2011;71:Abstract nr P1-12-19.
- Burris HA 3rd, Rugo HS, Vukelja SJ, et al. Phase II study of the antibody drug conjugate trastuzumab-DM1 for the treatment of human epidermal growth factor receptor 2 (HER2)-positive breast cancer after prior HER2-directed therapy. J Clin Oncol 2011;29:398-405. [PubMed]
- Krop IE, LoRusso P, Miller KD, et al. A phase II study of trastuzumab emtansine in patients with human epidermal growth factor receptor 2-positive metastatic breast cancer who were previously treated with trastuzumab, lapatinib, an anthracycline, a taxane, and capecitabine. J Clin Oncol 2012;30:3234-41. [PubMed]
- Verma S, Miles D, Gianni L, et al. Trastuzumab emtansine for HER2-positive advanced breast cancer. N Engl J Med 2012;367:1783-91. [PubMed]
- Krop IE, Kim SB, González-Martín A, et al. Trastuzumab emtansine versus treatment of physician’s choice for pretreated HER2-positive advanced breast cancer (TH3RESA): a randomised, open-label, phase 3 trial. Lancet Oncol 2014;15:689-99. [PubMed]
- Hurvitz SA, Dirix L, Kocsis J, et al. Phase II randomized study of trastuzumab emtansine versus trastuzumab plus docetaxel in patients with human epidermal growth factor receptor 2-positive metastatic breast cancer. J Clin Oncol 2013;31:1157-63. [PubMed]
- Amiri-Kordestani L, Blumenthal GM, Xu QC, et al. FDA approval: ado-trastuzumab emtansine for the treatment of patients with HER2-positive metastatic breast cancer. Clin Cancer Res 2014;20:4436-41. [PubMed]
- Press MF, Cordon-Cardo C, Slamon DJ. Expression of the HER-2/neu proto-oncogene in normal human adult and fetal tissues. Oncogene 1990;5:953-62. [PubMed]
- Thon JN, Devine MT, Jurak Begonja A, et al. High-content live-cell imaging assay used to establish mechanism of trastuzumab emtansine (T-DM1)--mediated inhibition of platelet production. Blood 2012;120:1975-84. [PubMed]
- Mahapatra K, Darbonne W, Bumbaca D, et al. Abstract A135: T-DM1-induced thrombocytopenia results from impaired platelet production in a HER2-independent manner. Mol Cancer Ther 2011;10:Abstract nr A135.
- Bender BC, Schaedeli-StarK F, Joshi A, et al. A semi-physiologic population pharmacokinetic/pharmacodynamic (PK/PD) model of thrombocytopenia (TCP) characterizing the effect of trastuzumab-DM1 (T-DM1) on platelet counts in patients with HER2-positive MBC. J Clin Oncol 2011;29:abstr 605.
- Perez EA, Hurvitz SA, Amler LC, et al. Relationship between HER2 expression and efficacy with first-line trastuzumab emtansine compared with trastuzumab plus docetaxel in TDM4450g: a randomized phase II study of patients with previously untreated HER2-positive metastatic breast cancer. Breast Cancer Res 2014;16:R50. [PubMed]
- LoRusso P, Krop IE, Burris III HA, et al. Quantitative assessment of diagnostic markers and correlations with efficacy in two phase II studies of trastuzumab-DM1 (T-DM1) for patients (pts) with metastatic breast cancer (MBC) who had progressed on prior HER2-directed therapy. J Clin Oncol 2010;28:abstr 1016.
- Kataoka Y, Mukohara T, Shimada H, et al. Association between gain-of-function mutations in PIK3CA and resistance to HER2-targeted agents in HER2-amplified breast cancer cell lines. Ann Oncol 2010;21:255-62. [PubMed]
- Baselga J, Verma S, Ro J, et al. Abstract LB-63: Relationship between tumor biomarkers (BM) and efficacy in EMILIA, a phase III study of trastuzumab emtansine (T-DM1) in HER2-positive metastatic breast cancer (MBC). Cancer Res 2013;73:Abstract nr LB-63.
- Phillips GD, Fields CT, Li G, et al. Dual targeting of HER2-positive cancer with trastuzumab emtansine and pertuzumab: critical role for neuregulin blockade in antitumor response to combination therapy. Clin Cancer Res 2014;20:456-68. [PubMed]
- Miller K, Gianni L, Andre F, et al. A phase Ib/II trial of trastuzumab-DM1 (T-DM1) with pertuzumab (P) for women with HER2-positive, locally advanced or metastatic breast cancer (BC) who were previously treated with trastuzumab (T). J Clin Oncol 2010;28:abstr 1012.
- Modi S, Elias AD, LoRusso P, et al. Results from a phase Ib study of trastuzumab emtansine (T-DM1), paclitaxel (T), and pertuzumab (P) in patients with HER2-positive metastatic breast cancer (MBC) previously treated with trastuzumab. J Clin Oncol 2012;30:abstr 528.
- Martin M, Dewar J, Albanell J, et al. Abstract P4-12-07: Neoadjuvant trastuzumab emtansine and docetaxel, with or without pertuzumab, in patients with HER2-positive early-stage breast cancer: Results from a phase 1b/2a study. Cancer Res 2013;73:Abstract nr P4-12-07.
- Denlinger CS, Beeram M, Tolcher AW, et al. A phase I/II and pharmacologic study of MM-111 in patients with advanced, refractory HER2-positive (HER2+) cancers. J Clin Oncol 2010;28:abstr TPS169.


