Diagnosis of Helicobacter pylori using the rapid urease test
Introduction
Helicobacter pylori (H. pylori) is an important human pathogen involved in the pathogenesis of atrophic gastritis, gastroduodenal ulcer, gastric cancer, MALT lymphoma, idiopathic thrombocytopenic purpura, iron deficiency anemia and vitamin B12 deficiency (1). Despite an overall decline in the prevalence of H. pylori infections in western countries, 30-40% of the USA population remains infected (2,3). H. pylori is not a commensal organism in that the infection always causes gastric mucosal inflammation and damage. The basic lesion is progressive mucosal inflammation which may result in preneoplastic atrophic changes (1). Although H. pylori vary in virulence (e.g., whether the cag pathogenicity island is present), there is a risk of a significant clinical outcome as the difference in risk between the least and the most virulent is only approximately twofold (1). Because the rate of progression of the mucosal damage is unpredictable, and the infection is always transmissible, it has been recommended that whenever an H. pylori infection is found it should be cured unless there are compelling reasons that would mitigate that choice (e.g., very elderly with extensive comorbid diseases) (4). In February on 2013, the Japanese government approved a universal test and treat strategy as part of Japan’s goal to eliminate gastric cancer (5).
H. pylori infections like other major chronic infectious diseases (i.e., syphilis and tuberculosis) are associated with a long latent period before presenting clinically. As such, many infections will be discovered during this latent period. A number of methods to H. pylori infection have been developed and they are generally grouped as being “invasive” meaning that they require gastric tissue or mucus, or “non-invasive” requiring only blood, breath or stool or analysis. Here, we discuss the rapid urease test (RUT) or RUT which is an invasive test in that it requires sampling of the gastric mucosa. The test provides indirect evidence of the infection by identifying the presence of a non-mammalian enzyme, urease, in or on the gastric mucosa.
History
H. pylori was first cultured in 1983. The initial report described it as urease-negative. However, other laboratories attempting to replicate the initial finding correctly identified the organism was urease positive (6). McNulty et al. adapted a standard laboratory test for urease activity in which a loop of culture is placed in a urea containing substrate with a pH indicator such as Christensen’s 2% urea broth; Urease hydrolyzes the urea to produce ammonia and carbon dioxide (7). The ammonia increases the pH leading to the color change (from brown to pink). McNulty et al. showed that urease could be identified directly from gastric biopsies using this test thus eliminating the need for culture or histology. The original observation was followed with a large clinical trial published in 1989 in which 1,445 patients underwent testing for H. pylori using modified Christensen’s urea broth (8). They reported almost 100% specificity and 96% sensitivity in comparison with histology and culture. Barry Marshal added an antibacterial agent to an agar gel containing urea and a pH indicator and patented the first test RUT called the campylobacter-like organism (CLO) test which rapidly has become a standard for clinical use and for clinical trials.
Gastric urease
Gastric urease had a long history prior to the discovery of H. pylori (9). For decades it was believed that urease was a mammalian enzyme and it was hypothesized that urease was produced by the stomach in order to protect the stomach from digestion by gastric acid (9). Urease was even the basis for peptic ulcer therapy. For example, Oliver Fitzgerald, an Irish physician, treated peptic ulcers with both oral and intravenously administered urea to neutralize gastric acidity and heal peptic ulcers (10). The first urea breath test equivalent using 13C-urea was actually reported in 1951 and used to measure urease activity in a frog’s stomach (11). When it became evident that urease was microbial in origin, interest in urease waned as it was clear it could not be responsible for protecting the gastric mucosa against damage by acid and because most of the pertinent experiments had already been done. The discovery that H. pylori produced abundant urease allowed these many experiments to assume new meaning (6).
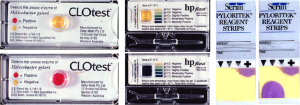
The RUT is an indirect test of the presence of H. pylori based on the presence of urease in or on the gastric mucosa. It has an advantage over serology in that it only detects the presence of an active infection. The test requires a sample of gastric mucosa or mucus that is added to a tube, gel, or other device which brings that sample into contact with urea and a method to detect the products of urea hydrolysis, ammonia or carbon dioxide. Different tests have been developed to assess the presence of gastric urease activity and have been based on probably every possible component of the reaction including those that assess changes in labeled CO2 in serum or breath, breath ammonia, and pH change induced by the ammonia either as a color change or directly using a pH meter. The initial test used phenol red in which the color indicator which changes from yellow to pink or red as the pH increases. Other tests have used different indicators each with a potential advantage such as being able to start the reaction at a lower pH such as 5.4 and thus reduce the activity of contaminating mouth bacteria many of which also contain abundant urease (e.g., HPfast®) (Figure 1). The simplest tests are home-made and contain a small quantity of substrate in a tube with a pH indicator and can be produced of one or two cents each (12).
As for any enzymatic reaction, one must consider the parameters that affect the reaction such as substrate concentration, enzyme concentration and activity, time, and temperature and studies have been done to test many of the different parameters so as to provide reliable results. The important variables include sample collection, sample size, time before scoring the test as negative, and whether warming is beneficial. A positive RUT requires approximately 105H. pylori in the biopsy sample to change the color using an agar-based test such as the CLO test (13). This is generally not a problem as the concentration of H. pylori typically exceeds that minimum. The sensitivity of various RUT tests as primary diagnostic tests is high and has been reported to vary between approximately 80% and 100% and specificity between 97% and 99% (14-17).
The first requirement is that the biopsy sample must come from a site where the organisms are present. Patients with duodenal ulcer typically have non-atrophic mucosa and H. pylori are especially abundant throughout the antrum. For those patients a single sample from the antrum or gastric angle will have a high yield, typically greater than 85% and often in the range of 95% to 100%. However, if the patient has atrophic gastritis and large areas of intestinal metaplasia, which contain few H. pylori organisms, the results will often be disappointing. Numerous studies of biopsy site have been published. For example, one study took two biopsy specimens from the antrum and reported a sensitivity and specificity of 95% and 100% (18). Another study compared the results from a biopsy taken at the gastric angle, a prepyloric site and a corpus site with results of 100%, 87% and 84.4%, respectively and no false-positive results (i.e., specificity 100%) (19). Overall, for best results, two samples, one from the antrum avoiding areas of ulceration and obvious intestinal metaplasia, and one from normal appearing corpus are sufficient and provide the highest yield. The two samples can be placed into the same gel or media or into separate containers depending on physician preference and whether other uses are planned for the samples.
Biopsy size
The sensitivity and specificity of jumbo cup forceps (3.3 mm diameter) and tiny cup forceps (1.8 mm diameter) were compared using antral biopsies from the same patients (20). The sensitivity and specificity with jumbo forceps were 92.1% and 95.5% compared to 88.5% and 98.8% for the tiny forceps, respectively. Thus, for practical purposes biopsy cup size seems to make little difference. Because larger forceps also provide more information for histologic examination, we recommend that the largest forceps that will fit through the insertion channel of the endoscopy be used. If a truly tiny endoscopy is used, such as an ultra-slim endoscope, samples for RUT can be taken using either the tiny forceps supplied or by brushing the mucosa and placing the brush in the RUT media.
Reaction speed of a RUT depends in part of the temperature of the reaction. A comparison was done in which antral biopsies were placed at 38 °C in a warmer or kept at room temperature (~21 °C). The ability to make a diagnosis within 30 minutes was 20% higher using the warmer (21) but overall the results were the same. Many endoscopists place the RUT device in their pocket to warm them. Overall, the use of warmer makes little difference except it may allow for a diagnosis to be confirmed more often before the patient leaves the endoscopic area.
Commercially available RUT kits suggest that the decision be made (positive vs. negative) within 24 hours. The time the test turns positive depends on the concentration of bacteria and the temperature. Most will turn positive within 120 to 180 minutes but it is best to hold those that appear negative for 24 hours (22,23). After 24 hours the test may turn positive from the presence non-H. pylori urease containing organisms (24). Positive results after 24 hours are most often false positive and should not be used for treatment decisions. We routinely discard the samples after 24 hours. There are a number of different commercially available RUT kits that primarily differ depending on the platform (e.g., gel, liquid, membrane, etc.). Choice depends on availability and local preference as none has proven to be superior.
False-negative and false-positive results
As noted above, the RUT is a test for the presence of the urease enzyme. The actual results will however depend on the gastric disease and the likelihood of atrophic changes or exogenous factors that reduce the bacterial load and thus produce false negative results. False positive results can occur if other urease containing organisms are present in sufficient quantity or if one allows contact of the specimen and the media for a prolonged period, typically longer than 24 hours. As noted before, approximately 105 bacteria must be present in the biopsy sample for a positive result (13) and anything that reduces the bacterial density such as the use of antibiotics, bismuth-containing compounds, or proton pump inhibitors may result in false-negative results (25-27). The two most common reasons for false negative results are the recent use of proton pump inhibitors and the presence of intestinal metaplasia. H2-receptor antagonists do not reduce the bacterial density and can be used up to the day of the test (28). It is unclear how long one must wait after stopping proton pump inhibitors before the possibility of a false negative result becomes unlikely. The recommendation of two weeks is commonly given based on being on the safe side; however the organisms typically recover rapidly from inhibition with a proton pump inhibitor (26,29). Nonetheless, all of the tests for active infection including RUT, histology, urea breath test, and culture may become false negative during proton pump inhibitor use or after bismuth or antibiotic use (28). It is unlikely that a false negative RUT will also be accompanied by histologically uninflamed and normal gastric mucosa. When in doubt and the result is important, it is best to obtain a noninvasive test (urea breath test or stool antigen) after discontinuation of the PPI.
False-positives are rare and when present may be due to the presence of other urease containing organisms such as Proteus mirabilis, Citrobactor freundii, Klebsiella pneumonia, Enterobactor cloacae and Staphylococcus aureus (22). However, unless the patient has achlorhydria or hypochlorhydria, non-H. pylori organisms are unlikely to be present in sufficient concentration to produce a positive test unless the RUT substrate lacks an inhibitor to bacterial growth in which they may possibly overgrow during the 24 hours observation period.
Clinical interpretation of the RUT results
The interpretation of RUT, like any diagnostic test, depends in part on the pretest probability of an infection (30). Thus, in a patient with a duodenal ulcer, a single positive RUT would be considered confirmative of the diagnosis whereas a negative test would need to confirm by the results of another test such as histology (e.g., no evidence of gastric inflammation). In contrast, in an elderly patient undergoing endoscopy for gastro-esophageal reflux disease, one would be hesitant to start therapy based only on the basis of a positive RUT. Thus, interpretation depends on the pretest probability and the importance of the result in relation to the subsequent management of the patient. In order to save the patient money, some physicians have suggested taking biopsies for RUT as well as for histology from normal appearing mucosa and then, if the RUT is positive, discarding the histology specimens as unnecessary (23,31,32). While this may make sense in the presence of a high pretest possibility (e.g., active duodenal ulcer), it would not be prudent in a patient with non-ulcer dyspepsia where the histologic findings themselves may be important (e.g., presence of atrophic changes, dysplasia, etc.). It has also been recommended that RUT should not be used as the sole arbiter of the results of H. pylori eradication therapy because the sensitivity of the RUT is not 100% and thus a negative test does not fully exclude the presence of an active infection (4,28). This is particularly important when the reason for the examination is for test-of-cure. In reality, endoscopy is infrequently done for test of cure except in patients with lesions where further endoscopic and/or histologic evaluation is needed such as gastric ulcer or after resection of adenomatous polyps and the question of the role of RUT post therapy rarely arises. For those patients it is probably most cost effective to rely on histologic examination of antral and corpus biopsies.
Generally speaking, upper endoscopy is an expensive test associated with a small but definite risk and unless there are specific contraindications, biopsy for examination of the mucosal histology is generally indicated. RUT testing can also be done and is especially helpful in difficult diagnostic situations when the physician would like to start treatment soon. As such biopsies for RUT are often taken in patient with recent upper gastrointestinal bleeding. In this situation, there may be an increased prevalence of false negative results. It has been postulated that blood leads to decrease sensitivity of RUT possibly related to the presence of albumin (33), H. pylori killing factors in human plasma (34), or blood in gastric lumen (35,36). However, other studies report that blood does not influence the test (37,38). False negative tests are also common after partial gastrectomy probably because of reduced bacterial load often related on the presence of bile (39,40). In none of these situations, it is critical to diagnose or start treatment for an H. pylori infection immediately. As such false negative results have little clinical importance unless they are accepted as proof of the absence of an H. pylori infection. One must always fall back on considering what one plans to do with the information (i.e., positive tests are generally to be believed but negative tests require histologic or other confirmation when the presence of an H. pylori infection is likely responsible for the reason for the endoscopy such as with a bleeding peptic ulcer). Since most H. pylori infections were acquired in childhood, there is little need to treat immediately and one can always wait until the acute conditions have resolved.
Use of the RUT sample for additional purposes
The tissue sample contained in the agar of an RUT test can be used for another purpose. For example the sample can be removed from the agar gel of positive tests and used for molecular testing for H. pylori and/or for the presence of clarithromycin resistance (41). Since the sample contains host tissue, it could also be used for other testing such as the CYP2C19 genotype of the host (42).
Summary
The RUT is a rapid, cheap and simple test that is used frequently in clinical practice. The RUT is best considered as a screening test and not as the gold standard for H. pylori infection. For clinical trials the diagnosis of an H. pylori infection requires that two different types of test be positive or a positive culture. H. pylori negative is typically defined as all tests negative. The key to proper use is to consider any test in terms of pretest probability as well as in the importance of the information in terms of patient management. False negative tests are more frequent than false positive tests and thus a negative result should not be used to exclude H. pylori when a wrong diagnosis would be detrimental to patient management (e.g., in a patient with recent upper gastrointestinal bleeding or after H. pylori eradication therapy). A positive culture is considered the only true gold standard for the diagnosis of H. pylori such that additional testing is needed when one suspects a false-negative result. Finally, the test can also be used as an informal assessment of the accuracy of the pathology laboratory and discrepancies between the RUT and histology especially a positive RUT and negative histology should prompt review of the histopathology and discussions with the pathologist.
Acknowledgements
Dr. Graham is supported in part by the Office of Research and Development Medical Research Service Department of Veterans Affairs, Public Health Service grants R01 DK062813 and DK56338 which funds the Texas Medical Center Digestive Diseases Center. The contents are solely the responsibility of the authors and do not necessarily represent the official views of the VA or NIH.
Authors’ contributions: Each of the authors has been involved equally and has read and approved the final manuscript. Each meets the criteria for authorship established by the International Committee of Medical Journal Editors and verifies the validity of the results reported.
Disclosure: Dr. Graham is an unpaid consultant for Novartis in relation to vaccine development for treatment or prevention of H. pylori infection. Dr. Graham is a paid consultant for RedHill Biopharma regarding novel H. pylori therapies and has received research support for culture of H. pylori. He is a consultant for Otsuka Pharmaceuticals regarding diagnostic breath testing. Dr. Graham has received royalties from Baylor College of Medicine patents covering materials related to 13C-urea breath test. Dr. Uotani has nothing to declare.
References
- Shiotani A, Cen P, Graham DY. Eradication of gastric cancer is now both possible and practical. Semin Cancer Biol 2013;23:492-501. [PubMed]
- Peterson WL, Fendrick AM, Cave DR, et al. Helicobacter pylori-related disease: guidelines for testing and treatment. Arch Intern Med 2000;160:1285-91. [PubMed]
- Grad YH, Lipsitch M, Aiello AE. Secular trends in Helicobacter pylori seroprevalence in adults in the United States: evidence for sustained race/ethnic disparities. Am J Epidemiol 2012;175:54-9. [PubMed]
- Malfertheiner P, Megraud F, O'Morain CA, et al. Management of Helicobacter pylori infection--the Maastricht IV/ Florence Consensus Report. Gut 2012;61:646-64. [PubMed]
- Asaka M, Kato M, Sakamoto N. Roadmap to eliminate gastric cancer with Helicobacter pylori eradication and consecutive surveillance in Japan. J Gastroenterol 2014;49:1-8. [PubMed]
- Campylobacter-like organisms in the stomach of patients and healthy individuals. Lancet 1984;1:1348-9. [PubMed]
- McNulty CA, Wise R. Rapid diagnosis of Campylobacter-associated gastritis. Lancet 1985;1:1443-4. [PubMed]
- McNulty CA, Dent JC, Uff JS, et al. Detection of Campylobacter pylori by the biopsy urease test: an assessment in 1445 patients. Gut 1989;30:1058-62. [PubMed]
- Graham DY, Go MF, Evans DJ Jr. Review article: urease, gastric ammonium/ammonia, and Helicobacter pylori--the past, the present, and recommendations for future research. Aliment Pharmacol Ther 1992;6:659-69. [PubMed]
- Fitzgerald O, Murphy P. Studies on the physiological chemistry and clinical significance of urease with special reference to the stomach. Irish J Med Sci 1950;292:97-159.
- Von Korff RW, Glick D. Role of urease in the gastric mucosa. II. In vitro studies with isotopic urea on frog mucosa. Am J Physiol 1951;165:688-94. [PubMed]
- Hazell SL, Borody TJ, Gal A, et al. Campylobacter pyloridis gastritis I: Detection of urease as a marker of bacterial colonization and gastritis. Am J Gastroenterol 1987;82:292-6. [PubMed]
- Mégraud F, Bessède E, Lehours P. Current methods used for the diagnosis of Helicobacter pylori infection. In: Buzás GM. eds. Helicobacter pylori - A Worldwide Perspective 2014. Oak Park: Bentham Science, 2014:234-58.
- Calvet X, Sánchez-Delgado J, Montserrat A, et al. Accuracy of diagnostic tests for Helicobacter pylori: a reappraisal. Clin Infect Dis 2009;48:1385-91. [PubMed]
- Al-Humayed SM, Ahmed ME, Bello CS, et al. Comparison of 4 laboratory methods for detection of Helicobacter pylori. Saudi Med J 2008;29:530-2. [PubMed]
- Redéen S, Petersson F, Törnkrantz E, et al. Reliability of Diagnostic Tests for Helicobacter pylori Infection. Gastroenterol Res Pract 2011;2011:940650.
- Vaira D, Perna F. How useful is the rapid urease test for evaluating the success of Helicobacter pylori eradication therapy? Nat Clin Pract Gastroenterol Hepatol 2007;4:600-1. [PubMed]
- el-Zimaity HM, al-Assi MT, Genta RM, et al. Confirmation of successful therapy of Helicobacter pylori infection: number and site of biopsies or a rapid urease test. Am J Gastroenterol 1995;90:1962-4. [PubMed]
- Woo JS, el-Zimaity HM, Genta RM, et al. The best gastric site for obtaining a positive rapid ureas test. Helicobacter 1996;1:256-9. [PubMed]
- Yousfi MM, El-Zimaity HM, Cole RA, et al. Detection of Helicobacter pylori by rapid urease tests: is biopsy size a critical variable? Gastrointest Endosc 1996;43:222-4. [PubMed]
- Yousfi MM, El-Zimaity HM, Cole RA, et al. Does using a warmer influence the results of rapid urease testing for Helicobacter pylori? Gastrointest Endosc 1996;43:260-1. [PubMed]
- Osaki T, Mabe K, Hanawa T, et al. Urease-positive bacteria in the stomach induce a false-positive reaction in a urea breath test for diagnosis of Helicobacter pylori infection. J Med Microbiol 2008;57:814-9. [PubMed]
- Perna F, Ricci C, Gatta L, et al. Diagnostic accuracy of a new rapid urease test (Pronto Dry), before and after treatment of Helicobacter pylori infection. Minerva Gastroenterol Dietol 2005;51:247-54. [PubMed]
- Vaira D, Holton J, Cairns S, et al. Urease tests for Campylobacter pylori: care in interpretation. J Clin Pathol 1988;41:812-3. [PubMed]
- Graham DY, Lew GM, Malaty HM, et al. Factors influencing the eradication of Helicobacter pylori with triple therapy. Gastroenterology 1992;102:493-6. [PubMed]
- Graham DY, Opekun AR, Hammoud F, et al. Studies regarding the mechanism of false negative urea breath tests with proton pump inhibitors. Am J Gastroenterol 2003;98:1005-9. [PubMed]
- Forné M, Viver JM, Espinós JC, et al. Impact of colloidal bismuth subnitrate in the eradication rates of Helicobacter pylori infection-associated duodenal ulcer using a short treatment regimen with omeprazole and clarithromycin: a randomized study. Am J Gastroenterol 1995;90:718-21. [PubMed]
- Attumi TA, Graham DY. Follow-up testing after treatment of Helicobacter pylori infections: cautions, caveats, and recommendations. Clin Gastroenterol Hepatol 2011;9:373-5. [PubMed]
- Laine L, Estrada R, Trujillo M, et al. Effect of proton-pump inhibitor therapy on diagnostic testing for Helicobacter pylori. Ann Intern Med 1998;129:547-50. [PubMed]
- Vecchio TJ. Predictive value of a single diagnostic test in unselected populations. N Engl J Med 1966;274:1171-3. [PubMed]
- Midolo P, Marshall BJ. Accurate diagnosis of Helicobacter pylori. Urease tests. Gastroenterol Clin North Am 2000;29:871-8. [PubMed]
- Laine L, Lewin D, Naritoku W, et al. Prospective comparison of commercially available rapid urease tests for the diagnosis of Helicobacter pylori. Gastrointest Endosc 1996;44:523-6. [PubMed]
- Leung WK, Sung JJ, Siu KL, et al. False-negative biopsy urease test in bleeding ulcers caused by the buffering effects of blood. Am J Gastroenterol 1998;93:1914-8. [PubMed]
- Houghton J, Ramamoorthy R, Pandya H, et al. Human plasma is directly bacteriocidal against Helicobacter pylori in vitro, potentially explaining the decreased detection of Helicobacter pylori during acute upper GI bleeding. Gastrointest Endosc 2002;55:11-6. [PubMed]
- Tu TC, Lee CL, Wu CH, et al. Comparison of invasive and noninvasive tests for detecting Helicobacter pylori infection in bleeding peptic ulcers. Gastrointest Endosc 1999;49:302-6. [PubMed]
- Wildner-Christensen M, Touborg Lassen A, Lindebjerg J, et al. Diagnosis of Helicobacter pylori in bleeding peptic ulcer patients, evaluation of urea-based tests. Digestion 2002;66:9-13. [PubMed]
- Tang JH, Liu NJ, Cheng HT, et al. Endoscopic diagnosis of Helicobacter pylori infection by rapid urease test in bleeding peptic ulcers: a prospective case-control study. J Clin Gastroenterol 2009;43:133-9. [PubMed]
- Archimandritis A, Tzivras M, Sougioultzis S, et al. Rapid urease test is less sensitive than histology in diagnosing Helicobacter pylori infection in patients with non-variceal upper gastrointestinal bleeding. J Gastroenterol Hepatol 2000;15:369-73. [PubMed]
- Tian XY, Zhu H, Zhao J, et al. Diagnostic performance of urea breath test, rapid urea test, and histology for Helicobacter pylori infection in patients with partial gastrectomy: a meta-analysis. J Clin Gastroenterol 2012;46:285-92. [PubMed]
- Adamopoulos AB, Stergiou GS, Sakizlis GN, et al. Diagnostic value of rapid urease test and urea breath test for Helicobacter pylori detection in patients with Billroth II gastrectomy: a prospective controlled trial. Dig Liver Dis 2009;41:4-8. [PubMed]
- Li Y, Rimbara E, Thirumurthi S, et al. Detection of clarithromycin resistance in Helicobacter pylori following noncryogenic storage of rapid urease tests for 30 days. J Dig Dis 2012;13:54-9. [PubMed]
- Furuta T, Sugimoto M, Kodaira C, et al. Sitafloxacin-based third-line rescue regimens for Helicobacter pylori infection in Japan. J Gastroenterol Hepatol 2014;29:487-93. [PubMed]


