Glucan supplementation enhances the immune response against an influenza challenge in mice
Introduction
β1,3-D-glucans (hereafter referred to as “glucans”) form part of a group of natural biologically active materials generally called immunomodulators or biological response modifiers. Glucans are conserved carbohydrates forming structural components of cell walls of yeast, fungi, seaweed, and cereals. Generally, the term glucan is sometimes used as a chemical name of glucose polymer and represents a group of chemically heterogeneous carbohydrates consisting of various numbers of glucose molecules bound together in various types of linkages.
The various biological effects of glucans are already well established, and include various effects such as potentiation of anti-infectious immunity, inhibition of cancer growth, reduction of stress, or reduction of cholesterol level (1,2). So far, glucans have been found to be active in a wide range of species, but no animal species have been found to be glucan-resistant. In addition, the immunostimulating effects of glucans have been examined in human models. Soluble glucan was found to decrease the incidence of infection and the need for antibiotics (3). Recently, glucan was used as part of a vaccine for high risk neuroblastoma, and the results were encouraging enough (4) to warrant phase II clinical trials. Glucan supplement reduced upper respiratory symptoms and improved the mood state in stressed women (5). Jesenak’s group showed immunomodulatory effects of mushroom-derived glucan in children with respiratory infections (6). In addition, a series of clinical studies showed strong effects on the treatment of children with chronic respiratory problems (7,8). Therefore, it is not surprising that glucan has been widely used in Japan for the treatment of gastrointestinal cancer (9).
Influenza infection represents a serious health problem worldwide and approximately half a million people die annually (10). Recently, the focus of several investigations was on the effects of various immunomodulators on the outcome of influenza infection. Zheng et al. showed that delayed antiviral plus immunomodulator treatment reduced mortality in mice (11), which was later supported by similar results from the Cardenas-Freytag group (12). In some studies, immunomodulators enhanced the protective effects of the flu vaccine (13). In a swine model, glucan showed strong antiviral effects on influenza infection via production of nitric oxide and interferon gamma (14). In addition, glucan isolated from Aureobasidium pullulans was effective in the prevention of influenza (15). Based on these results, we have chosen a murine model to investigate the role of a glucan formulation on changes in immune reaction caused by the influenza infection.
Materials and methods
Animals
Female, 8-week-old BALB/c mice were purchased from the Jackson Laboratory (Bar Harbor, ME, USA). The protocol for the research project has been approved by the University of Louisville IACUC Committee and it conforms to the provisions of the Declaration of Helsinki (as revised in Edinburgh 2000). Animals were sacrificed by CO2 asphyxiation followed by cervical dislocation.
Phagocytosis
Phagocytosis of synthetic polymeric microspheres was described earlier (16). Briefly, 0.1 mL of peripheral blood from glucan-treated or untreated mice (both groups were infected with influenza) was incubated in vitro with 0.05 mL of 2-hydroxyethyl methacrylate particles (HEMA; 5×108/mL). The tubes were incubated at 37 °C for 60 min, with intermittent shaking. Smears were stained with Wright stain (Sigma). The cells with three or more HEMA particles were considered positive. Mice were injected with either glucan or PBS (control). All experiments were performed in triplicate. At least 300 cells were examined in each experiment.
Glucan
The glucan formulation used in this study consisted of a proprietary blend of MaitakeGold 404 maitake fruit body extract and whole mycelial mushroom powders of Shiitake, Reishi, Agaricus, and Chaga mushrooms. Each of these glucan sources were chosen for inclusion in the blend based on their ability to enhance immune function and provide resistance to viral infection. The Shiitake, Reishi, Agaricus, and Chaga mushrooms used in this study are alpha- and beta-glucan-rich whole mushroom powders obtained from Gourmet Mushrooms, Inc., (Sebastopol, CA, USA) which are grown on brown rice, dried, and then ground into a fine powder. MaitakeGold 404 was produced under a patented method (USA, Patent 5,854,404). The product, which is a glucan/protein complex, is derived by thermally extracting the fruit body of Maitake with water under pressure at 100 °C or more for 30 min to an hour. After that, alcohol is added to the extract at a final concentration of 20% to 60% by volume to remove floating material by filtration. The resulting extract is concentrated under heating to remove residual alcohol. The product is a hygroscopic powder in shades of brown, which is soluble in water, alkaline solutions and dimethyl sulfoxide, with a molecular weight around 1,000 kD. The final daily dose in the combined glucan formulation was 881.5 µg of glucan.
Plaque assay
Plaque assay for monitoring virus titers of lung homogenates was performed as described previously (17). Briefly, 10% suspensions of the lung homogenates were examined. Serial dilutions of the samples were inoculated on Madin-Darby canine kidney cells, overlaid with RPMI 1640 medium containing 1% Bacto Agar, incubated for 48 h and enumerated.
Antibody titer
Anti-influenza hemagglutination-specific antibodies in serum were measured by ELISA following a previously described protocol (18). A purified hemagglutination protein was used for plate coating at 2 mg/L concentration.
The virus challenge to mice
Mice were orally treated with the glucan mixture or PBS once a day for 14 days by gavage. At day 14, the same mice were intranasally challenged with the H5N1 A/HK/483 influenza virus (1,000 50% mouse infectious dose diluted in PBS to a 50 µL volume) as described previously (19). Mice were monitored daily for morbidity and measured for survival, body weight changes and body temperature (using CODA, Ken Scientific, Torrington, CT, USA). The samples were immediately frozen and stored at –80 °C for subsequent determination.
Quantification of cytokines
Tissue homogenates were analyzed for the levels of IL-1β, TNF-α and IFN-γ by use of ELISA kits (R&D Systems, Minneapolis, MN, USA) according to the manufacturer’s instructions.
In vitro cytotoxicity assay
Cells were isolated from the spleens of tested mice by standard methods. Cell suspension was generated by pressing minced spleen against the bottom of a petri dish containing PBS. After elimination of erythrocytes by 10-second incubation in distilled water and five washes in cold PBS, the cells were resuspended in PBS and counted. The viability was determined by trypan blue exclusion and only cells with viability better than 95% were used in subsequent experiments. Splenocytes (106/mL; 0.1 mL/well) in V-shaped 96-well microplates were then washed three times with RPMI 1640 medium. After washing, 50 µL of target cell line K562 (ATCC, Manassas, VA, USA) was added. After spinning the plates at 250 × g for 5 min, the plates were incubated for 4 h at 37 °C. The cytotoxic activity of cells was determined by the use of CytoTox 96 Non-Radioactive Cytotoxicity Assay according to the manufacturer’s instructions. Briefly, 10 µL of lysis solution was added into appropriate control wells 45 min before the end of incubation. The next step was to spin the plates at 250 × g for 5 min, followed by transferring 50 µL of supernatant into flat-bottomed, 96-well microplates. After 50 µL of reconstituted substrate was added into each well, plates were covered and incubated for 30 min at room temperature in the dark. The optical density was determined by using a STL ELISA reader (Tecan U.S., Research Triangle Park, NC, USA) at 492 nm. Specific cell-mediated cytotoxicity was calculated using the formula:
Percent-specific killing (%, cytotoxicity)=100× [(OD492 experimental − OD492 spontaneous) divided (OD492 maximum − OD492 spontaneous)] as described in the manufacturer’s instructions where spontaneous release was target cells incubated with medium alone and maximum release was obtained from target cells lysed with the solution provided in the kit.
Results
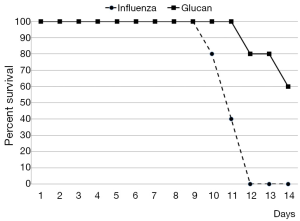
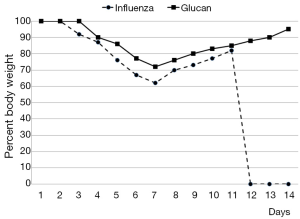
By day 12 post-infection, all PBS-treated mice had succumbed, while 60% of glucan-treated mice survived (Figure 1). Furthermore, control mice started to regain weight by day 7, but at day 12 (when all died) they still did not reach the weight of the glucan-treated group, which achieved normal weight around day 14 (Figure 2).
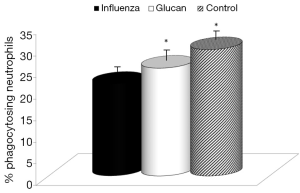
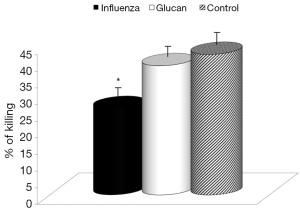
First, we focused on effects of immune reactions. In the case of glucan, first attention was usually devoted to cellular immunity in general, and to phagocytosis in particular. We used an established model of synthetic microspheres with low spontaneous adherence to the cell membrane and showed that glucan treatment significantly restored the phagocytic activity of peripheral blood neutrophils that was reduced by viral infection (Figure 3). Similarly, treatment with glucan returned the NK cell activity to normal values after infection with the virus. Data presented in Figure 4 are from an effector/target ratio of 1:50, but two additional ratios (1:10 and 1:100) offered similar results (data not shown), showed that these effects are not based on a specific effector/target ratio.
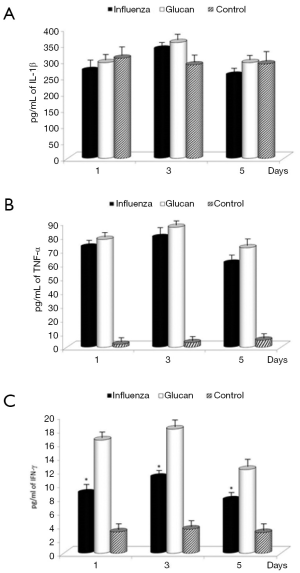
To determine the proinflammatory cytokine levels following influenza infection in mice, lungs from mice were collected on days 1, 3 and 5 and homogenates were subsequently assayed for IL-1β, TNF-α, and IFN-γ levels by ELISA. Influenza infection in mice receiving PBS resulted in increasing levels of TNF-α and IFN-γ compared to control mice, illustrating the normal immune reaction to infection. Levels of IFN-γ were further significantly stimulated by supplementation with glucan. Glucan treatment resulted in a small additional increase in IL-1β on day 3 and TNF-α vs. the influenza plus PBS treatment. The levels of all cytokines in glucan-treated mice were greater when compared to untreated (control) mice, except on day 1 in the case of IL-1β. The treatment with glucan caused an increase in these levels, which in the case of IFN-γ was statistically significant (Figure 5).
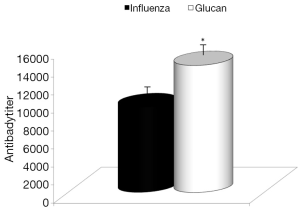
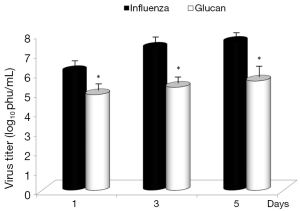
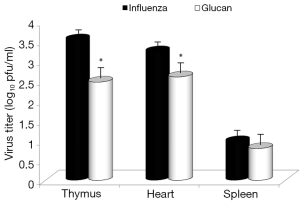
Dietary addition of glucan for 14 days significantly potentiated the antibody response to influenza (Figure 6). Similar effects were found when we measured the levels of antibodies in earlier time intervals (data not shown). To further investigate the effect of oral administration of glucan on the viral replication, we monitored virus titers in the lungs by plaque assay. As shown in Figure 7, the results indicate that although the virus titers were not different between infected mice fed PBS and infected glucan-treated animals on day 1, the titers significantly decreased in treated mice from day 3 after infection. Data shown in Figure 8 revealed that consumption of glucan significantly lowered the virus titer in the thymus and heart.
Discussion
In this study, we focused on a unique formulation of glucan used as a dietary supplement to test its effects on resistance to influenza infection, which is one of the most infectious human agents. Influenza epidemics occur every year in the United States and worldwide.
The formulation consisted of a mixture of various glucans, some of which were previously shown to have significant effects on immune reactions (20,21). Glucans are generally considered to be stimulators of cellular immunity; therefore, it is not surprising that phagocytosis and NK cell activity are two most commonly studied reactions. A study of Miyazaki’s group suggested that glucan treatment might be effective for the prevention of influenza in animals, so we decided to evaluate the effects of this unique glucan mixture on influenza infection (15).
To further investigate the effects of oral administration of this glucan mixture on the prevention of influenza, first we monitored the rate of survival and loss of body weight after infection with influenza virus. The survival rate of mice orally administered *glucan significantly increased, with 60% of mice living on day 14 (vs. 100% mortality in the control group). Loss of body weight was significantly lower in glucan-treated mice than in untreated mice, with similar effects on body temperature.
The systemic effects of glucan on a wide range of immune reactions are well established (1,2). The effects on phagocytosis are particularly well known (16). At the same time, viral infections are known to disrupt various aspects of the immune system (22). Our findings showed that supplementation with glucan can return the influenza-depressed phagocytic activity to almost normal levels, which correspond with known effects of glucans towards immunosuppression caused by various immunotoxic substances (23). Similar reactions were also studied using glucan derived from Aureobasidium pullulans. The effects of glucan on inflammatory cytokines differed from those found in our study (15), which is most probably caused by a completely different glucan and by a different method of cytokine detection.
Influenza infection affects a cascade of immune functions, including phagocytosis, production of antibodies and various cytokines. Some of these cytokines have strong immunomodulatory effects, while others are more associated with the symptoms and pathology of infection. Following influenza infection, a wide spectrum of cytokines is produced in numerous organs, including lymph nodes, bronchoalveoli and spleen (24,25).
Most individual cytokines are pleiotropic and often even redundant in their functions. It is thus rather difficult to determine the individual contribution of the particular cytokine produced after influenza infection. However, it is believed that IFN-γ has a protective role against infection, whereas IL-1, TNF-α and IL-6 are involved in the inflammatory phase.
We established that there were no substantial differences in IL-1β levels between PBS and glucan-treated groups after viral infection. In the case of TNF-γ, infection resulted in a substantial increase in cytokine production for both groups; however, there was almost no production of this cytokine in control untreated (and uninfected) animals. The situation with IFN-γ was different, as glucan treatment caused a significant increase of IFN-γ secretion in all tested intervals. The kinetics of IL-1 and IFN-γ changes caused by influenza corresponded to the previously found data (19).
Consistent with widely known facts about glucan and antibody stimulation (5,16), glucan supplementation increased antibody titers stimulated by influenza infection. Again, these findings are consistent with smaller weight loss and lower morbidity in glucan-supplemented mice. Similarly, glucan treatment enhanced immune function, resulting in lower virus titers in the thymus and heart.
However, it is important to note that since between-glucan variations exist for many measures of efficacy, the use of proprietary blends of glucan sources may result in reduced efficacy for a given combination. For a review of sources of between-glucan variations, see (26).
Conclusions
In summary, the current study showed that dietary glucan can significantly reduce the effects of influenza infection. Lower mortality and overall effects of infection are most probably affected by stimulation of both cellular and humoral responses leading to the lower viral load in many organs. These results suggest that consumption of dietary glucan might be potentially useful as a complementary or alternative approach to treatment of influenza infection.
Acknowledgements
Disclosure: The authors declare no conflict of interest.
References
- Vetvicka V. eds. β-Glucans as Natural Biological Response Modifiers. New York: Nova Science Publishers, 2013.
- Vetvicka V, Novak M. eds. Biology and Chemistry of Beta Glucan, Vol. 2, Beta-glucan, Structure, Chemistry and Specific Application. Oak Park: Bentham Science Publisher, 2013.
- Babineau TJ, Marcello P, Swails W, et al. Randomized phase I/II trial of a macrophage-specific immunomodulator (PGG-glucan) in high-risk surgical patients. Ann Surg 1994;220:601-9. [PubMed]
- Kushner BH, Cheung IY, Modak S, et al. Phase I trial of a bivalent gangliosides vaccine in combination with β-glucan for high-risk neuroblastoma in second or later remission. Clin Cancer Res 2014;20:1375-82. [PubMed]
- Talbott SM, Talbott JA. Baker's yeast beta-glucan supplement reduces upper respiratory symptoms and improves mood state in stressed women. J Am Coll Nutr 2012;31:295-300. [PubMed]
- Jesenak M, Majtan J, Rennerova Z, et al. Immunomodulatory effect of pleuran (β-glucan from Pleurotus ostreatus) in children with recurrent respiratory tract infections. Int Immunopharmacol 2013;15:395-9. [PubMed]
- Vetvicka V, Richter J, Svozil V, et al. Placebo-driven clinical trials of yeast-derived β-(1-3) glucan in children with chronic respiratory problems. Ann Transl Med 2013;1:26. [PubMed]
- Richter J, Svozil V, Král V, et al. Clinical trials of yeast-derived β-(1,3) glucan in children: effects on innate immunity. Ann Transl Med 2014;2:15. [PubMed]
- Ina K, Kataoka T, Ando T. The use of lentinan for treating gastric cancer. Anticancer Agents Med Chem 2013;13:681-8. [PubMed]
- Stöhr K. Influenza--WHO cares. Lancet Infect Dis 2002;2:517. [PubMed]
- Zheng BJ, Chan KW, Lin YP, et al. Delayed antiviral plus immunomodulator treatment still reduces mortality in mice infected by high inoculum of influenza A/H5N1 virus. Proc Natl Acad Sci U S A 2008;105:8091-6. [PubMed]
- Norton EB, Clements JD, Voss TG, et al. Prophylactic administration of bacterially derived immunomodulators improves the outcome of influenza virus infection in a murine model. J Virol 2010;84:2983-95. [PubMed]
- Du X, Wang J, Niu X, et al. Dietary wolfberry supplementation enhances the protective effect of flu vaccine against influenza challenge in aged mice. J Nutr 2014;144:224-9. [PubMed]
- Jung K, Ha Y, Ha SK, et al. Antiviral effect of Saccharomyces cerevisiae beta-glucan to swine influenza virus by increased production of interferon-gamma and nitric oxide. J Vet Med B Infect Dis Vet Public Health 2004;51:72-6. [PubMed]
- Muramatsu D, Iwai A, Aoki S, et al. β-Glucan derived from Aureobasidium pullulans is effective for the prevention of influenza in mice. PLoS One 2012;7:e41399. [PubMed]
- Vetvicka V, Vetvickova J. β-1,3-Glucan: silver bullet or hot air? Open Glycoscience 2010;3:1-6.
- Takada A, Matsushita S, Ninomiya A, et al. Intranasal immunization with formalin-inactivated virus vaccine induces a broad spectrum of heterosubtypic immunity against influenza A virus infection in mice. Vaccine 2003;21:3212-8. [PubMed]
- Wen Z, Ye L, Gao Y, et al. Immunization by influenza virus-like particles protects aged mice against lethal influenza virus challenge. Antiviral Res 2009;84:215-24. [PubMed]
- Szretter KJ, Gangappa S, Lu X, et al. Role of host cytokine responses in the pathogenesis of avian H5N1 influenza viruses in mice. J Virol 2007;81:2736-44. [PubMed]
- Vetvicka V, Vetvickova J. Immune-enhancing effects of Maitake (Grifola frondosa) and Shiitake (Lentinula edodes) extracts. Ann Transl Med 2014;2:14. [PubMed]
- Vetvicka V, Vetvickova J. Immune enhancing effects of WB365, a novel combination of Ashwagandha (Withania somnifera) and Maitake (Grifola frondosa) extracts. N Am J Med Sci 2011;3:320-4. [PubMed]
- Iwasaki A, Pillai PS. Innate immunity to influenza virus infection. Nat Rev Immunol 2014;14:315-28. [PubMed]
- Vetvicka V, Vetvickova J. Glucan–resveratrol–vitamin C combination offers protection against toxic agents. Toxins (Basel) 2012;4:1301-8. [PubMed]
- Hoeve MA, Nash AA, Jackson D, et al. Influenza virus A infection of human monocyte and macrophage subpopulations reveals increased susceptibility associated with cell differentiation. PLoS One 2012;7:e29443. [PubMed]
- Han SN, Meydani SN. Antioxidants, cytokines, and influenza infection in aged mice and elderly humans. J Infect Dis 2000;182:S74-80. [PubMed]
- Story J, Vetvicka V, Angove M. Beta1,3-glucan anticancer efficacies and synergies: a review. Am J Immunol 2014;10:131-43.


