Minimally invasive procedures
Introduction
Spontaneous pneumothorax is the presence of air in the pleural cavity. Pneumothorax is classified as primary and secondary. The secondary due to the underlying disease. The primary spontaneous pneumothorax, is a common disease amongst young people, with an incidence of 18-28/100,000 a year, in men and 1,2-6/100,000 in women (1).
The primary spontaneous pneumothorax needs hospitalization and frequent surgical therapy, mainly due to the increased possibility of relapse (16-52%) after the first incident (2,3). The primary spontaneous pneumothorax surgical therapy consists of excision of the cysts and pleurodesis, which is the most effective prevention method for possible relapses. Video-assisted thoracoscopic surgery (VATS) as well as axillary thoracotomy are considered to be acceptable surgical approaches in handling primary spontaneous pneumothorax according to the instructions of the American College of Chest Physicians as well as the British Thoracic Society (4,5). Even though the VATS is preferred over open surgeries, because it reduces the hospitalization and is less painful, it has a bigger, by four times, relapse frequency (6,7). The open surgery with a small axillary thoracotomy is considered to be a safe and acceptable method of treating with spontaneous pneumothorax, with a satisfactory cosmetic result and a small relapse frequency.
Surgical technique
Patients with a relapsing spontaneous pneumothorax, are initially treated by placing a tube drainage of the half thorax rib cage, usually 24-French. They are then submitted to a high definition computed tomography in order to define the possible cause of pneumothorax. According to the indication, they prepare for surgical treatment.
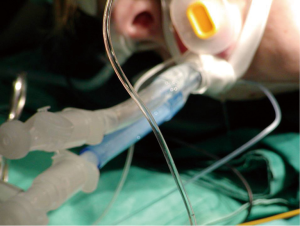
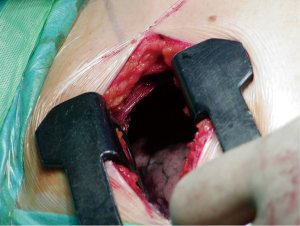
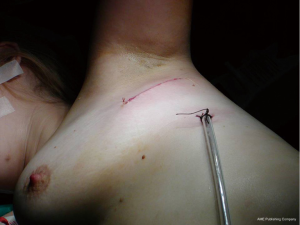
During surgery, under general anesthesia, the patients are intubated with a double lumen endotracheal tube. They are then positioned for axillary thoracotomy extending the shoulder so that the axillary is fully revealed. The area is disinfected using an antiseptic solution and the area is covered with surgical settings. An approximately 6 cm skin incision is applied on the axillary area, between the posterior limit of the pectoralis major muscle and the anterior limit of the vastus dorsi muscle. The hemithorax is approached via the 3rd intercostal space. Entering the diseased hemithorax is assisted by using a special expander. The neurovascular bundle (intercostal vein, intercostal artery and intercostal nerve) is protected by placing gauze in between the speculum and the lower edge of the third side. The pneumothorax causes (usually cysts) are dissected using endo-GIA 45-mm by putting suture line reinforcement. Then it is carefully detached and the parietal pleura are dissected from the top of the lung, up to the fourth intercostal space. Then the lung is expanded, and using a normal saline that is infused in the hemithorax, it is checked for possible air leakage. A 24-French thorascotomy tube is inserted in the hemithorax at the midaxillary line and pushed to the top of the lung where it is stabilized by a No1 silk stitching. Three trapeze shaped 1-0 vicryl peripleural sutura are usually placed.
The trauma is then joined per layer with 2-0 vicryl while for the intradermal suturing, we use 3-0 vicryl rapide. The thoracostomy tube is connected with a negative suction system (−15-−20 cmH2O) and remains in that position for 48 more hours even if there is no air leakage. The tube is removed when there is no air leakage, when the lung is fully expanded at the chest X−ray and when the supply doesn’t exceed 100 mL/24 h. The patients leave the hospital the day after the tube is removed (Figures 1,2).

Our experience on 124 patients with primary spontaneous pneumothorax
Within the last 8 years 124 consecutive patients with spontaneous pneumothorax were admitted to our department. There were 84 males and 40 females, ranging in age from 18 to 67 years. Primary indications for operation were recurrent spontaneous pneumothorax in 92 patients and persistent air leak in 32 patients. All patients underwent a limited axillary thoracotomy Blebs or bullae were found in all patients and were ablated by stapling. Extended pleural abrasion was also performed.
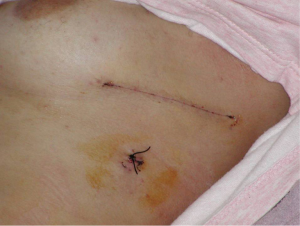
The average operative time (measured from the time of the skin incision until leaving the operating theatre) was 54 mm. There were 12 postoperative complications including wound infection in four patients, fever in four, re-operation in two patients due to bleeding and shoulder arthritis in two patients (rate 9.6%).The duration of use of the thoracostomy tube after surgery for all 124 patients averaged 2.6 days, and postoperative hospitalization averaged 5.4 days. All showed good lung expansion postoperatively. Perioperative mortality was zero. No patient had a recurrence of a pneumothorax on the operated side at an average of 32 months following their axillary thoracotomy. There was universal satisfaction with the size and positioning of the scar, with patients finding it extremely acceptable cosmetically.
VATS versus thoracotomy in the surgical treatment of spontaneous pneumothorax
Surgical management of PSP is usually indicated in patients with recurrent ipsilateral pneumothorax, first episode with occupational risk or persistent air leakage. A first episode of a PSP is treated by observation if the area of pneumothorax is <20% or by simple aspiration if >20%, but recurrences are frequent. For recurrent or persisting pneumothorax, a more invasive surgical approach is indicated. There are two objectives in the surgical management of pneumothorax. The first widely accepted objective is resection of blebs or the suture of apical perforations to treat the underlying defect. The second objective is to create a pleural symphysis to prevent recurrence. The procedure can be approached through open thoracotomy or VATS (8) (Figures 3,4).
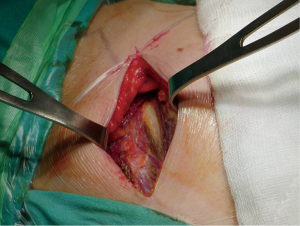
The classical posterolateral thoracotomy is now rarely used for the treatment of the primary spontaneous pneumothorax. This standard thoracotomy has been replaced by smaller incisions as axillary thoracotomy, anterior thoracotomy, muscle-sparing lateral thoracotomy, and a variety of posterior, lateral and axillary mini-thoracotomy procedures. The median sternotomy for simultaneous treatment of both lungs is performed only in, 1% of cases (9-13). There have been few prospective clinical trials comparing VATS and thoracotomy in the interventional treatment of spontaneous pneumothorax.
Waller et al., compared the results on 30 patients underwent bullectomy and apical pleurectomy by VATS, performed through three 2-cm incisions and on 30 patients underwent a similar surgical procedure through a posterolateral thoracotomy and concluded that VATS is superior, (regard to the postoperative pain, hospital stay, and pulmonary dysfunction), to thoracotomy in the treatment of primary spontaneous pneumothorax (14). Crisci et al., considered retrospectively the results obtained in a series of 30 consecutive patients with recurrent spontaneous pneumothorax treated with VATS between November 1991 and August 1994 in comparison with those obtained in a group of 30 patients previously treated with a traditional thoracotomy. The authors demonstrated that in VATS the total economic cost is lower about 22.7%, in comparison with traditional thoracotomy (15).
Kim and colleagues, on 66 patients with recurrent, persistent or contralateral spontaneous pneumothorax underwent surgical therapy (36 patients were treated by VATS and 30 by transaxillary minithoracotomy) conclude that there were no advantages of VATS over transaxillary minithoracotomy regard to the operating time, the amount of analgesics used on the first postoperative day, the duration of the indwelling chest tube, and the number of postoperative recurrences in patients with apical bullae (16). Atta et al. in their study in 1997, with small number of the patients, only 9, concluded that VAT is a safe and reasonably effective treatment of spontaneous pneumothorax (17). Horio et al., in their important study, in 1998, on 97 patients with spontaneous pneumothorax underwent resection of pulmonary bullae by VATS (51 patients) or limited axillary thoracotomy (44 patients) argue strongly that the pneumothorax recurrence rate in VATS cases was double that in limited axillary thoracotomy cases and that the main reason for recurrence is the cysts and bullae that escape the attention of the surgeon (18). The retrospective study of Al-Qudah et al. was designed to compare the contribution of the VATSand open thoracotomy in the management of spontaneous pneumothorax. The authors recorded the results of surgical treatment in 100 patients and concluded that VATS have been shown to produce results comparable to those obtained following open thoracotomy with reduction of postoperative pain, respiratory dysfunction, catabolic response to trauma and decrease in wound related complications and VATS should be used with caution for the management of secondary pneumothorax (19). Freixinet et al. in their prospective randomized study on 90 patients with primary spontaneous pneumothorax underwent surgical treatment (46 patients by VATS and 44 patients by axillary thoracotomy) demonstrate that Video-assisted thoracoscopy and axillary thoracotomy offer similar results (20). Barker et al., in their work reviewed the literature for studies on pneumothorax surgery (four randomised and 25 non-randomised) and the authors concluded that both randomised and non-randomised trials are consistent in recurrence of pneumothoraces and show a four-fold increase with a video-assisted approach compared with an open approach (21). Vohra and colleagues in their interesting study conclude that VATS pleurectomy has been shown to be comparable to open pleurectomy in the treatment of spontaneous pneumothorax, with a meta-analysis and several prospective randomised controlled trial showing reductions in length of hospital stay and analgesic requirements. Postoperative pulmonary dysfunction has also been shown to be reduced after VATS pleurectomy (22). Balduyck et al., in an effort to evaluate quality of life (QoL) evolution after VATS and anterolateral thoracotomy for primary and secondary spontaneous pneumothorax studied prospectively 20 consecutive patients, using the European Organization for Research and Treatment of Cancer (EORTC) QoL Questionnaire-C30 and the lung specific module LC-13. The researchers demonstrated that pneumothorax surgery is well tolerated by the majority of patients. In general, patients QoL subscales improved after surgery. After VATS, pain, dyspnoea and thoracic pain decreased significantly. After anterolateral thoracotomy, a significant increase was observed in general QoL, physical and emotional functioning. Dyspnoea and coughing decreased after anterolateral thoracotomy. Both techniques were comparable in QoL evolution. However, one month after surgery, physical, role, cognitive functioning and dyspnoea were significantly better in the VATS group. VATS also had a favourable thoracic pain evolution compared to anterolateral thoracotomy (23). Foroulis et al., in the interesting prospective randomized study on 66 patients underwent surgical intervention for recurrent spontaneous pneumothorax through either a modified two-port VATS procedure (33 patients) or axillary minithoracotomy (33 patients) demonstrate that the recurrence rate, complication rate, postoperative chest tube drainage duration, postoperative hospital stay, and incidence of chronic pain did not differ between the two groups but VATS seems to offers to the patient more satisfaction (24). Fatimi et al. in their study on 39 patients presented with spontaneous pneumothorax and had undergone VATS, reported a recurrence rate of 7.6% (25). Joshi and colleagues in a recent publication on 163 patients underwent surgical intervention for pneumothorax (86 patients underwent VATS under a single surgeon with extensive VATS experience while 79 patients underwent an open procedure) noted that there was no statistically significant difference in the recurrence rate between the open and the VATS group (1% vs. 3.5%, P=1.0) and concluded that the VATS group was superior to the open group in terms of reduced postoperative bleeding (7.5% vs. 0%, P=0.01), reduced number of intensive care unit admissions (16% vs. 0%, P<0.01) and a reduced adjusted length of stay (3 vs. 5.5 days, P<0.01) (26-45). These data demonstrated that VATS offers a shorter postoperative hospital stay, less postoperative pain, improved pulmonary gas exchange postoperatively, lower total economic cost but has a higher recurrence rate of approximately 5% (46-65). In conclusion, open thoracotomy (with minimally invasive techniques) remains the procedure with lowest recurrence rate (approximately 1%) for simple cases and for difficult or recurrent pneumothoraces (59,66-75). Therefore the patients about to undergo surgery for pneumothorax should be informed in detail about the pros and cons of interventions (Figures 5,6).
Acknowledgements
Disclosure: The authors declare no conflict of interest.
References
- Henry M, Arnold T, Harvey J, et al. BTS guidelines for the management of spontaneous pneumothorax. Thorax 2003;58 Suppl 2:ii39-52. [PubMed]
- Melton LJ 3rd, Hepper NG, Offord KP. Incidence of spontaneous pneumothorax in Olmsted County, Minnesota: 1950 to 1974. Am Rev Respir Dis 1979;120:1379-82. [PubMed]
- Wait MA, Estrera A. Changing clinical spectrum of spontaneous pneumothorax. Am J Surg 1992;164:528-31. [PubMed]
- Baumann MH, Strange C, Heffner JE, et al. Management of spontaneous pneumothorax: an American College of Chest Physicians Delphi consensus statement. Chest 2001;119:590-602. [PubMed]
- Miller AC, Harvey JE. Guidelines for the management of spontaneous pneumothorax. Standards of Care Committee, British Thoracic Society. BMJ 1993;307:114-6. [PubMed]
- Chan SS. Current opinions and practices in the treatment of spontaneous pneumothorax. J Accid Emerg Med 2000;17:165-9. [PubMed]
- Al-Alawi M, Subramaniam A, Khan R, et al. Management of primary spontaneous pneumothorax: an audit into practice. Ir Med J 2013;106:62. [PubMed]
- Luh SP. Review: Diagnosis and treatment of primary spontaneous pneumothorax. J Zhejiang Univ Sci B 2010;11:735-44. [PubMed]
- Murray KD, Matheny RG, Howanitz EP, et al. A limited axillary thoracotomy as primary treatment for recurrent spontaneous pneumothorax. Chest 1993;103:137-42. [PubMed]
- Simansky DA, Yellin A. Pleural abrasion via axillary thoracotomy in the era of video assisted thoracic surgery. Thorax 1994;49:922-3. [PubMed]
- Körner H, Andersen KS, Stangeland L, et al. Surgical treatment of spontaneous pneumothorax by wedge resection without pleurodesis or pleurectomy. Eur J Cardiothorac Surg 1996;10:656-9. [PubMed]
- Donahue DM, Wright CD, Viale G, et al. Resection of pulmonary blebs and pleurodesis for spontaneous pneumothorax. Chest 1993;104:1767-9. [PubMed]
- Athanassiadi K, Kalavrouziotis G, Loutsidis A, et al. Surgical treatment of spontaneous pneumothorax: ten-year experience. World J Surg 1998;22:803-6. [PubMed]
- Waller DA, Forty J, Morritt GN. Video-assisted thoracoscopic surgery versus thoracotomy for spontaneous pneumothorax. Ann Thorac Surg 1994;58:372-6. [PubMed]
- Crisci R, Coloni GF. Video-assisted thoracoscopic surgery versus thoracotomy for recurrent spontaneous pneumothorax. A comparison of results and costs. Eur J Cardiothorac Surg 1996;10:556-60. [PubMed]
- Kim KH, Kim HK, Han JY, et al. Transaxillary minithoracotomy versus video-assisted thoracic surgery for spontaneous pneumothorax. Ann Thorac Surg 1996;61:1510-2. [PubMed]
- Atta HM, Latouf O, Moore JE, et al. Thoracotomy versus video-assisted thoracoscopic pleurectomy for spontaneous pneumothorax. Am Surg 1997;63:209-12. [PubMed]
- Horio H, Nomori H, Fuyuno G, et al. Limited axillary thoracotomy vs video-assisted thoracoscopic surgery for spontaneous pneumothorax. Surg Endosc 1998;12:1155-8. [PubMed]
- Al-Qudah A. Video-assisted thoracoscopy versus open thoracotomy for spontaneous pneumothorax. J Korean Med Sci 1999;14:147-52. [PubMed]
- Freixinet JL, Canalís E, Juliá G, et al. Axillary thoracotomy versus videothoracoscopy for the treatment of primary spontaneous pneumothorax. Ann Thorac Surg 2004;78:417-20. [PubMed]
- Barker A, Maratos EC, Edmonds L, et al. Recurrence rates of video-assisted thoracoscopic versus open surgery in the prevention of recurrent pneumothoraces: a systematic review of randomised and non-randomised trials. Lancet 2007;370:329-35. [PubMed]
- Vohra HA, Adamson L, Weeden DF. Does video-assisted thoracoscopic pleurectomy result in better outcomes than open pleurectomy for primary spontaneous pneumothorax? Interact Cardiovasc Thorac Surg 2008;7:673-7. [PubMed]
- Balduyck B, Hendriks J, Lauwers P, et al. Quality of life evolution after surgery for primary or secondary spontaneous pneumothorax: a prospective study comparing different surgical techniques. Interact Cardiovasc Thorac Surg 2008;7:45-9. [PubMed]
- Foroulis CN, Anastasiadis K, Charokopos N, et al. A modified two-port thoracoscopic technique versus axillary minithoracotomy for the treatment of recurrent spontaneous pneumothorax: a prospective randomized study. Surg Endosc 2012;26:607-14. [PubMed]
- Fatimi SH, Hanif HM, Aziz S, et al. How VATS has changed the management of spontaneous pneumothorax in the 21st century. J Pak Med Assoc 2012;62:1041-5. [PubMed]
- Joshi V, Kirmani B, Zacharias J. Thoracotomy versus VATS: is there an optimal approach to treating pneumothorax? Ann R Coll Surg Engl 2013;95:61-4. [PubMed]
- Isaka T, Takahashi K, Maehara T, et al. Intraoperative core needle biopsy under complete video-assisted thoracic surgery for indeterminate tumor of lung. Surg Endosc 2015. [Epub ahead of print]. [PubMed]
- Maskell N; British Thoracic Society Pleural Disease Guideline Group. British Thoracic Society Pleural Disease Guidelines--2010 update. Thorax 2010;65:667-9. [PubMed]
- MacDuff A, Arnold A, Harvey J, et al. Management of spontaneous pneumothorax: British Thoracic Society Pleural Disease Guideline 2010. Thorax 2010;65 Suppl 2:ii18-31. [PubMed]
- Isaka M, Asai K, Urabe N. Surgery for secondary spontaneous pneumothorax: risk factors for recurrence and morbidity. Interact Cardiovasc Thorac Surg 2013;17:247-52. [PubMed]
- Kioumis IP, Zarogoulidis K, Huang H, et al. Pneumothorax in cystic fibrosis. J Thorac Dis 2014;6:S480-7. [PubMed]
- Kuhajda I, Zarogoulidis K, Kougioumtzi I, et al. Tube thoracostomy; chest tube implantation and follow up. J Thorac Dis 2014;6:S470-9. [PubMed]
- Manika K, Kioumis I, Zarogoulidis K, et al. Pneumothorax in sarcoidosis. J Thorac Dis 2014;6:S466-9. [PubMed]
- Kuhajda I, Zarogoulidis K, Kougioumtzi I, et al. Penetrating trauma. J Thorac Dis 2014;6:S461-5. [PubMed]
- Visouli AN, Zarogoulidis K, Kougioumtzi I, et al. Catamenial pneumothorax. J Thorac Dis 2014;6:S448-60. [PubMed]
- Huang Y, Huang H, Li Q, et al. Transbronchial lung biopsy and pneumothorax. J Thorac Dis 2014;6:S443-7. [PubMed]
- Terzi E, Zarogoulidis K, Kougioumtzi I, et al. Acute respiratory distress syndrome and pneumothorax. J Thorac Dis 2014;6:S435-42. [PubMed]
- Boskovic T, Stojanovic M, Stanic J, et al. Pneumothorax after transbronchial needle biopsy. J Thorac Dis 2014;6:S427-34. [PubMed]
- Li Z, Huang H, Li Q, et al. Pneumothorax: observation. J Thorac Dis 2014;6:S421-6. [PubMed]
- Huang Y, Huang H, Li Q, et al. Approach of the treatment for pneumothorax. J Thorac Dis 2014;6:S416-20. [PubMed]
- Chung JH, Choi YS, Cho JH, et al. Uniportal video-assisted thoracoscopic lobectomy: an alternative to conventional thoracoscopic lobectomy in lung cancer surgery? Interact Cardiovasc Thorac Surg 2015. [Epub ahead of print]. [PubMed]
- Browning RF, Parrish S, Sarkar S, et al. Bronchoscopic interventions for severe COPD. J Thorac Dis 2014;6:S407-15. [PubMed]
- Machairiotis N, Kougioumtzi I, Dryllis G, et al. Laparoscopy induced pneumothorax. J Thorac Dis 2014;6:S404-6. [PubMed]
- Ouellette DR, Parrish S, Browning RF, et al. Unusual causes of pneumothorax. J Thorac Dis 2014;6:S392-403. [PubMed]
- Parrish S, Browning RF, Turner JF Jr, et al. The role for medical thoracoscopy in pneumothorax. J Thorac Dis 2014;6:S383-91. [PubMed]
- Terzi E, Zarogoulidis K, Kougioumtzi I, et al. Human immunodeficiency virus infection and pneumothorax. J Thorac Dis 2014;6:S377-82. [PubMed]
- Zarogoulidis P, Kioumis I, Pitsiou G, et al. Pneumothorax: from definition to diagnosis and treatment. J Thorac Dis 2014;6:S372-6. [PubMed]
- Tsakiridis K, Mpakas A, Kesisis G, et al. Lung inflammatory response syndrome after cardiac-operations and treatment of lornoxicam. J Thorac Dis 2014;6 Suppl 1:S78-98. [PubMed]
- Tsakiridis K, Zarogoulidis P, Vretzkakis G, et al. Effect of lornoxicam in lung inflammatory response syndrome after operations for cardiac surgery with cardiopulmonary bypass. J Thorac Dis 2014;6 Suppl 1:S7-20. [PubMed]
- Argiriou M, Kolokotron SM, Sakellaridis T, et al. Right heart failure post left ventricular assist device implantation. J Thorac Dis 2014;6 Suppl 1:S52-9. [PubMed]
- Madesis A, Tsakiridis K, Zarogoulidis P, et al. Review of mitral valve insufficiency: repair or replacement. J Thorac Dis 2014;6 Suppl 1:S39-51. [PubMed]
- Siminelakis S, Kakourou A, Batistatou A, et al. Thirteen years follow-up of heart myxoma operated patients: what is the appropriate surgical technique? J Thorac Dis 2014;6 Suppl 1:S32-8. [PubMed]
- Foroulis CN, Kleontas A, Karatzopoulos A, et al. Early reoperation performed for the management of complications in patients undergoing general thoracic surgical procedures. J Thorac Dis 2014;6 Suppl 1:S21-31. [PubMed]
- Nikolaos P, Vasilios L, Efstratios K, et al. Therapeutic modalities for Pancoast tumors. J Thorac Dis 2014;6 Suppl 1:S180-93. [PubMed]
- Koutentakis M, Siminelakis S, Korantzopoulos P, et al. Surgical management of cardiac implantable electronic device infections. J Thorac Dis 2014;6 Suppl 1:S173-9. [PubMed]
- Spyratos D, Zarogoulidis P, Porpodis K, et al. Preoperative evaluation for lung cancer resection. J Thorac Dis 2014;6 Suppl 1:S162-6. [PubMed]
- Porpodis K, Zarogoulidis P, Spyratos D, et al. Pneumothorax and asthma. J Thorac Dis 2014;6 Suppl 1:S152-61. [PubMed]
- Panagopoulos N, Leivaditis V, Koletsis E, et al. Pancoast tumors: characteristics and preoperative assessment. J Thorac Dis 2014;6 Suppl 1:S108-15. [PubMed]
- Visouli AN, Darwiche K, Mpakas A, et al. Catamenial pneumothorax: a rare entity? Report of 5 cases and review of the literature. J Thorac Dis 2012;4 Suppl 1:17-31. [PubMed]
- Zarogoulidis P, Chatzaki E, Hohenforst-Schmidt W, et al. Management of malignant pleural effusion by suicide gene therapy in advanced stage lung cancer: a case series and literature review. Cancer Gene Ther 2012;19:593-600. [PubMed]
- Papaioannou M, Pitsiou G, Manika K, et al. COPD assessment test: a simple tool to evaluate disease severity and response to treatment. COPD 2014;11:489-95. [PubMed]
- Boskovic T, Stanic J, Pena-Karan S, et al. Pneumothorax after transthoracic needle biopsy of lung lesions under CT guidance. J Thorac Dis 2014;6 Suppl 1:S99-107. [PubMed]
- Papaiwannou A, Zarogoulidis P, Porpodis K, et al. Asthma-chronic obstructive pulmonary disease overlap syndrome (ACOS): current literature review. J Thorac Dis 2014;6 Suppl 1:S146-51. [PubMed]
- Zarogoulidis P, Porpodis K, Kioumis I, et al. Experimentation with inhaled bronchodilators and corticosteroids. Int J Pharm 2014;461:411-8. [PubMed]
- Bai C, Huang H, Yao X, et al. Application of flexible bronchoscopy in inhalation lung injury. Diagn Pathol 2013;8:174. [PubMed]
- Zarogoulidis P, Kioumis I, Porpodis K, et al. Clinical experimentation with aerosol antibiotics: current and future methods of administration. Drug Des Devel Ther 2013;7:1115-34. [PubMed]
- Zarogoulidis P, Pataka A, Terzi E, et al. Intensive care unit and lung cancer: when should we intubate? J Thorac Dis 2013;5:S407-12. [PubMed]
- Hohenforst-Schmidt W, Petermann A, Visouli A, et al. Successful application of extracorporeal membrane oxygenation due to pulmonary hemorrhage secondary to granulomatosis with polyangiitis. Drug Des Devel Ther 2013;7:627-33. [PubMed]
- Zarogoulidis P, Kontakiotis T, Tsakiridis K, et al. Difficult airway and difficult intubation in postintubation tracheal stenosis: a case report and literature review. Ther Clin Risk Manag 2012;8:279-86. [PubMed]
- Zarogoulidis P, Tsakiridis K, Kioumis I, et al. Cardiothoracic diseases: basic treatment. J Thorac Dis 2014;6 Suppl 1:S1. [PubMed]
- Kolettas A, Grosomanidis V, Kolettas V, et al. Influence of apnoeic oxygenation in respiratory and circulatory system under general anaesthesia. J Thorac Dis 2014;6 Suppl 1:S116-45. [PubMed]
- Turner JF, Quan W, Zarogoulidis P, et al. A case of pulmonary infiltrates in a patient with colon carcinoma. Case Rep Oncol 2014;7:39-42. [PubMed]
- Machairiotis N, Stylianaki A, Dryllis G, et al. Extrapelvic endometriosis: a rare entity or an under diagnosed condition? Diagn Pathol 2013;8:194. [PubMed]
- Tsakiridis K, Zarogoulidis P. An interview between a pulmonologist and a thoracic surgeon-Pleuroscopy: the reappearance of an old definition. J Thorac Dis 2013;5 Suppl 4:S449-51. [PubMed]
- Huang H, Li C, Zarogoulidis P, et al. Endometriosis of the lung: report of a case and literature review. Eur J Med Res 2013;18:13. [PubMed]