Prophylactic antibiotic administration for post cardiothoracic surgery sternal wounds: a retrospective study
Introduction
Cardiothoracic surgery procedures of the sternum are technically difficult. The skin which is the natural barrier against infection due to the incision breaks and a post surgical infection can occur. This type of infection is called surgical site infection (SSI) because it occurs on the part of the body where the surgery took place. One out of three patients tends to develop an SSI. There are three types of incisional infections: (I) superficial incisional SSI. This infection occurs just in the area of the skin where the surgical incision was made; (II) deep incisional SSI. This infection takes place beneath the incision area within the muscle tissue and fascia along with the surrounding the muscles.
Moreover; there is also the organ or space SSI. It is a type of infection that can occur in any area of the body such as; the skin, muscle, and fascia that was involved in the surgery or space between organs. There are signs and symptoms SSIs, these are: (I) redness; (II) tenderness; (III) fever; (IV) warmth; (V) pain; (VI) delayed healing and (VII) swelling (1,2). Furthermore: there are additional signs and symptoms for specific types of SSIs, these are summarized to the following: (I) locally to the site of the incision pus, or “purulent discharge”, from the wound site. In this case material from the wound site should be sent for culture in order to find out the types of germs that are causing the infection; (II) a deep incisional SSI may also produce pus. In this case the wound site may reopen on its own, or a surgeon may reopen the wound and find purulent discharge inside the wound; (III) there is also the case where an abscess is formed and pus is discharged form an organ or space of SSI. In this case the pus is drained through the drainage placed through the skin into a body space or organ. An abscess may be seen when the surgeon reopens the wound or by special X-ray studies (3,4). Usually infections after surgery are caused by germs called microorganisms. The most common of these include the bacteria Pseudomonas, Staphylococcus and Streptococcus (5). Microorganisms can infect a surgical wound through various forms of contact, such as from the touch of a contaminated caregiver or surgical instrument, through microorganisms in the air, or through microorganisms that are already on or in your body and then spread into the wound. Therefore pus culture is not always enough to determine the primary site of infection and blood or instrument parts should be also sent for cultures. Severity of an SSI is associated with the type of surgical wound and be classified as follows: (I) clean wounds. These are not inflamed or contaminated and do not involve operating on an internal organ; the risk for an SSI in this type of wound is less than 2 percent; (II) clean-contaminated wounds. These have no evidence of infection at the time of surgery, but do involve operating on an internal organ; the risk for SSI is less than 10 percent; (III) contaminated wounds. These involve operating on an internal organ with a spilling of contents from the organ into the wound; the risk for SSI is 13 to 20 percent and (IV) dirty wounds (6,7). These are wounds in which a known infection is present at the time of the surgery; the risk for SSI is about 40 percent (8,9). There are other additional risk factors for SSIs which can be summarized as follows: (I) commorbitities (diabetes, cancer, smoking habit, overweight and elderly); (II) immunesupression and (III) abdominal surgery (10). Prevention is of most importance and therefore investigation for a prophylactic antibiotic should be pursued. Until now cessation of smoking and prevention of shaving the possible operating area has been used proposed and followed by most patients. Diabetes mellitus should be also properly regulated and in any case the treating physician has to be quickly informed if the previously signs of infection are observed. SSIs can be treated with antibiotic medications (11-13). Sometimes additional surgery or procedures may be required to treat the SSI (14). In our study we investigated retrospectively which antibiotic efficiently prevented post surgery sterna wound infection and based on our findings we make a proposal.
Patients and methods
Five hundred and thirty five patients were enrolled from June 2012 to August 2013. The study was approved by our investigational review board (IRB) (“G. Papanikolaou” General Hospital, Theesaloniki, Greece). The purpose was to identify which one of the antibiotics administered protected the patients against post sternal infection or efficiently treated the post surgery infection (vancomycin, sultamicilin, ciprofloxacin, daptomycin, linezolide, teicoplanine and oxacilline). The following data were recorded from each patient: (I) days of hospitalization in the intensive care unit (ICU); (II) commorditites (underlying respiratory disease, diabetes etc.); (III) time under extracorpolar oxygenation (ECMO); (IV) usage of vacuum assisted closure; (V) body mass index (BMI); (VI) smoking habit; (VII) euroscore II; (VIII) cardiologic evaluation (NYHA score/heart ultrasound); (IX) usage of Intra-aortic balloon pump (IABP); (X) blood transfusion; (XI) renal failre and hemodialysis; (XII) multi-organ failure; (XIII) port surgery respiratory infection or sepsis; (XIV) days of hospitalization before surgery; (XV) place of transferal after surgery; (XVI) time in surgery; (XVII) second operation due to complications and (XVIII) record of microorganism isolation of the sterna wound. Euroscore has been previously published and validated (15,16).
Results
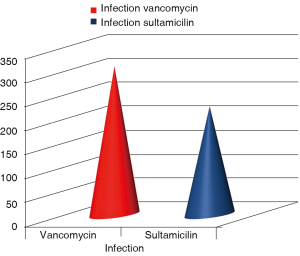
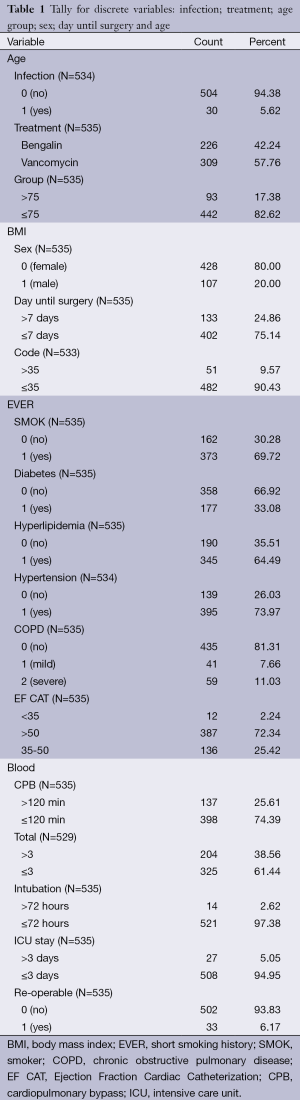
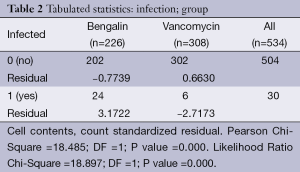
Chi-square analysis revealed a significant interaction effect between infection type and antibiotic (P<0.001) (Tables 1,2). In specific; it was observed that patients to whom vancomycin was administered had less post surgery infection than those to whom begalin was administered. Based on this “condition”, the treatment variable (vancomycin/sultamicilin) was regressed against all the demographic medical history and laboratory results. Male who were treated with vancomycin it was observed that they had 1.67 chances to be treated properly than female. It can be speculated that vancomycin can be used as a prophylactic treatment. Moreover; it was observed that patients with an ejection fraction of more than 30% had a rapid treatment period. Patients which were hospitalized for more than 7 days before surgery had 62.6% higher chances for post surgery infection. When cardiopulmonary bypass (CPB) was <120 min then a higher chance for post surgery infection was observed. Finally the two following observations were made. First; when the pre-white blood count number was >3,000 patients had a higher probability of post-surgery infection and secondly; when the white blood was <6,000 patients had higher probability for rapid treatment when post-surgery infection was observed.
Full table
Full table
Discussion
Cardiothoracic surgeries are considered stressful operations and special preparation is needed by the physicians. Sternal incision also is an invasive trauma and caution has to taken in order to avoid local infection. Based on our findings we propose that vancomycin can be used as an antibiotic prophylactic treatment. This statement comes in accordance with a previous observation made in our hospital. In specific we investigated the level of MRSA resistant staphylococcus and it was observed that it was 50%. Therefore at least for our hospital we will suggest vancomycin antibiotic as a prophylactic treatment against infection of the sterna wound (Figure 1). Vancomycin belongs to the glycopeptide antibiotic class and is effective mostly against Gram-positive bacteria. The original indication for vancomycin was for the treatment of penicillin-resistant Staphylococcus aureus. Vancomycin is primarily used for the treatment of serious infections caused by Gram-(+) bacteria known or suspected to be resistant to other antibiotics. The Infectious Disease Society of America recommends vancomycin as a first-line treatment for complicated skin infections, bloodstream infections, endocarditis, bone and joint infections, and meningitis infections caused by methicillin-resistant S. aureus (17). Orally administered vancomycin is recommended as a treatment for intestinal infection with Clostridium difficile, a common side effect of treatment with broad-spectrum antibiotics (18). Vancomycin is indicated for the treatment of serious, life-threatening infections by Gram-positive bacteria unresponsive to other less-toxic antibiotics. In particular, vancomycin should not be used to treat methicillin-sensitive Staphylococcus aureus because it is inferior to penicillins such as nafcillin (19). Although vancomycin levels are usually monitored, in an effort to reduce adverse events, the value of this is not beyond debate (20). Peak and trough levels are usually monitored, and, for research purposes, the area under the concentration curve is also sometimes used. Toxicity is best monitored by looking at trough values (21). Common adverse drug reactions (≥1% of patients) associated with IV vancomycin include: local pain, which may be severe, and thrombophlebitis. Damage to the kidneys and to the hearing were a side effect of the early impure versions of vancomycin, and these were prominent in the clinical trials conducted in the mid-1950s (22,23). Later trials using purer forms of vancomycin found nephrotoxicity is an infrequent adverse effect (0.1-1% of patients), but this is accentuated in the presence of aminoglycosides (24). Rare adverse effects (<0.1% of patients) include: anaphylaxis, toxic epidermal necrolysis, erythema multiforme, red man syndrome, superinfection, thrombocytopenia, neutropenia, leukopenia, tinnitus, and dizziness and/or ototoxicity (24). Vancomycin can induce platelet-reactive antibodies in the patient, leading to severe thrombocytopenia and bleeding with florid petechial hemorrhages, ecchymoses, and wet purpura (25). Vancomycin has traditionally been considered a nephrotoxic and ototoxic drug, based on observations by early investigators of elevated serum levels in renally impaired patients who had experienced ototoxicity, and subsequently through case reports in the medical literature. However, as the use of vancomycin increased with the spread of MRSA beginning in the 1970s, the previously reported rates of toxicity were recognized as not being observed. This was attributed to the removal of the impurities present in the earlier formulation of the drug, although those impurities were not specifically tested for toxicity (23). Plasma level monitoring of vancomycin is necessary due to the drug’s biexponential distribution, intermediate hydrophilicity, and potential for ototoxicity and nephrotoxicity, especially in populations with poor renal function and/or increased propensity to bacterial infection. Vancomycin activity is considered to be time-dependent; that is, antimicrobial activity depends on the duration that the serum drug concentration exceeds the minimum inhibitory concentration of the target organism. Thus, peak serum levels have not been shown to correlate with efficacy or toxicity; indeed, concentration monitoring is unnecessary in most cases. Circumstances in which therapeutic drug monitoring is warranted include: patients receiving concomitant aminoglycoside therapy, patients with (potentially) altered pharmacokinetic parameters, patients on haemodialysis, patients administered high-dose or prolonged treatment, and patients with impaired renal function. In such cases, trough concentrations are measured (26,27). Target ranges for serum vancomycin concentrations have changed over the years. Early authors suggested peak levels of 30-40 mg/L and trough levels of 5-10 mg/L, but current recommendations are that peak levels need not be measured and that trough levels of 10-15 or 15-20 mg/L (28), depending on the nature of the infection and the specific needs of the patient, may be appropriate (29,30). A few Gram-positive bacteria are intrinsically resistant to vancomycin: Leuconostoc and Pediococcus species, but these organisms rarely cause diseases in humans. Most Lactobacillus species are also intrinsically resistant to vancomycin, with the exception of L. acidophilus and L. delbruekii, which are sensitive. Other Gram-positive bacteria with intrinsic resistance to vancomycin include Erysipelothrix rhusiopathiae, Weissella confusa, and Clostridium innocuum. Most Gram-negative bacteria are intrinsically resistant to vancomycin because their outer membrane is impermeable to large glycopeptide molecules (with the exception of some non-gonococcal Neisseria species) (31-35). Evolution of microbial resistance to vancomycin is a growing problem, in particular, within healthcare facilities such as hospitals. While newer alternatives to vancomycin exist, such as linezolid [2000] and daptomycin [2003], the widespread use of vancomycin makes resistance to the drug a significant worry, especially for individual patients if resistant infections are not quickly identified and the patient continues the ineffective treatment. Vancomycin-resistant Enterococcus emerged in 1987. Vancomycin resistance evolved in more common pathogenic organisms during the 1990s and 2000s, including vancomycin-intermediate Staphylococcus aureus (VISA) and vancomycin-resistant Staphylococcus aureus (VRSA). Agricultural use of avoparcin, another similar glycopeptide antibiotic, may have contributed to the evolution of vancomycin-resistant organisms (36-40). Moreover; in our study it was observed that patients that remained more than seven days hospitilised prior to surgery had a higher rate of developing sterna wound infection, we attribute this finding to the high percentage or epidemiology of MRSA observed in our hospital. The elevated ejection fraction was observed to be positively associated with early treatment of infection, we attribute this finding to the more efficient circulation of the antibiotics to the region of the surgery. Finally, the white blood count level was an early indicator for infection. The higher the level before surgery (>3,000) the higher the chances were for developing infection). Finally, it was observed that when the CPB time was less than 120 minutes, there were less chances of developing infection, which we attribute to the better circulation of blood. We suggest based on our findings that an MRSA investigation should be performed in each surgery department and after patient data collection a case by case prophylactic antibiotic treatment should be applied before each surgery with sterna wound.
Acknowledgements
Disclosure: The authors declare no conflict of interest.
References
- Davis JS, Kourliouros A, Deshpande R, et al. Novel technique for avoidance of pressure competition between a negative pressure wound therapy device and chest drains in the management of deep sternal wound infections. Interact Cardiovasc Thorac Surg 2015;20:270-2. [PubMed]
- Tanaka D, Pitcher HT, Cavarocchi NC, et al. Can procalcitonin differentiate infection from systemic inflammatory reaction in patients on extracorporeal membrane oxygenation? J Heart Lung Transplant 2014;33:1186-8. [PubMed]
- Kelava M, Robich M, Houghtaling PL, et al. Hospitalization before surgery increases risk for postoperative infections. J Thorac Cardiovasc Surg 2014;148:1615-1621.e3.
- Wendler O, Baghai M. Infections post-cardiac surgery: new information during challenging times. J Am Coll Cardiol 2014;64:382-4. [PubMed]
- Kapoor R, Barnett CJ, Gutmann RM, et al. Preoperative Prevalence of Staphylococcus aureus in Cardiothoracic and Neurological Surgical Patients. Front Public Health 2014;2:204. [PubMed]
- Mukherjee K, Chakrabart U, Mazumder P, et al. Infected internal jugular vein thrombus in a case of infected arterio-venous fistula for dialysis access. Ann Vasc Dis 2014;7:335-8. [PubMed]
- Dohmen PM, Markou T, Ingemansson R, et al. Use of incisional negative pressure wound therapy on closed median sternal incisions after cardiothoracic surgery: clinical evidence and consensus recommendations. Med Sci Monit 2014;20:1814-25. [PubMed]
- Keshavamurthy S, Koch CG, Fraser TG, et al. Clostridium difficile infection after cardiac surgery: prevalence, morbidity, mortality, and resource utilization. J Thorac Cardiovasc Surg 2014;148:3157-65.e1-5.
- Marks DJ, Hyams C, Koo CY, et al. Clinical features, microbiology and surgical outcomes of infective endocarditis: a 13-year study from a UK tertiary cardiothoracic referral centre. QJM 2015;108:219-29. [PubMed]
- Robich MP, Schiltz N, Johnston DR, et al. Outcomes of patients with human immunodeficiency virus infection undergoing cardiovascular surgery in the United States. J Thorac Cardiovasc Surg 2014;148:3066-73. [PubMed]
- Ng RR, Myat Oo A, Liu W, et al. Changing glucose control target and risk of surgical site infection in a Southeast Asian population. J Thorac Cardiovasc Surg 2015;149:323-8. [PubMed]
- Lee P, Min L, Mody L. Perioperative Glucose Control and Infection Risk in Older Surgical Patients. Curr Geriatr Rep 2014;3:48-55. [PubMed]
- Gorski A, Hamouda K, Özkur M, et al. Cardiac surgery antibiotic prophylaxis and calculated empiric antibiotic therapy. Asian Cardiovasc Thorac Ann 2015;23:282-8. [PubMed]
- Gelijns AC, Moskowitz AJ, Acker MA, et al. Management practices and major infections after cardiac surgery. J Am Coll Cardiol 2014;64:372-81. [PubMed]
- Roques F, Nashef SA, Michel P, et al. Risk factors and outcome in European cardiac surgery: analysis of the EuroSCORE multinational database of 19030 patients. Eur J Cardiothorac Surg 1999;15:816-22; discussion 822-3. [PubMed]
- Roques F, Michel P, Goldstone AR, et al. The logistic EuroSCORE. Eur Heart J 2003;24:881-2. [PubMed]
- Atmaca O, Zarakolu P, Karahan C, et al. Risk factors and antibiotic use in methicillin-resistant Staphylococcus aureus bacteremia in hospitalized patients at Hacettepe University Adult and Oncology Hospitals (2004-2011) and antimicrobial susceptibilities of the isolates: a nested case-control study. Mikrobiyol Bul 2014;48:523-37. [PubMed]
- Gonzalez N, Sevillano D, Alou L, et al. Influence of the MBC/MIC ratio on the antibacterial activity of vancomycin versus linezolid against methicillin-resistant Staphylococcus aureus isolates in a pharmacodynamic model simulating serum and soft tissue interstitial fluid concentrations reported in diabetic patients. J Antimicrob Chemother 2013;68:2291-5. [PubMed]
- Small PM, Chambers HF. Vancomycin for Staphylococcus aureus endocarditis in intravenous drug users. Antimicrob Agents Chemother 1990;34:1227-31. [PubMed]
- Ackerman BH. Clinical value of monitoring serum vancomycin concentrations. Clin Infect Dis 1994;19:1180-2. [PubMed]
- Lodise TP, Patel N, Lomaestro BM, et al. Relationship between initial vancomycin concentration-time profile and nephrotoxicity among hospitalized patients. Clin Infect Dis 2009;49:507-14. [PubMed]
- Moellering RC Jr. Vancomycin: a 50-year reassessment. Clin Infect Dis 2006;42 Suppl 1:S3-4. [PubMed]
- Levine DP. Vancomycin: a history. Clin Infect Dis 2006;42 Suppl 1:S5-12. [PubMed]
- Farber BF, Moellering RC Jr. Retrospective study of the toxicity of preparations of vancomycin from 1974 to 1981. Antimicrob Agents Chemother 1983;23:138-41. [PubMed]
- Von Drygalski A, Curtis BR, Bougie DW, et al. Vancomycin-induced immune thrombocytopenia. N Engl J Med 2007;356:904-10. [PubMed]
- Moellering RC Jr. Monitoring serum vancomycin levels: climbing the mountain because it is there? Clin Infect Dis 1994;18:544-6. [PubMed]
- Karam CM, McKinnon PS, Neuhauser MM, et al. Outcome assessment of minimizing vancomycin monitoring and dosing adjustments. Pharmacotherapy 1999;19:257-66. [PubMed]
- Geraci JE. Vancomycin. Mayo Clin Proc 1977;52:631-4. [PubMed]
- Rybak M, Lomaestro B, Rotschafer JC, et al. Therapeutic monitoring of vancomycin in adult patients: a consensus review of the American Society of Health-System Pharmacists, the Infectious Diseases Society of America, and the Society of Infectious Diseases Pharmacists. Am J Health Syst Pharm 2009;66:82-98. [PubMed]
- Thomson AH, Staatz CE, Tobin CM, et al. Development and evaluation of vancomycin dosage guidelines designed to achieve new target concentrations. J Antimicrob Chemother 2009;63:1050-7. [PubMed]
- Swenson JM, Facklam RR, Thornsberry C. Antimicrobial susceptibility of vancomycin-resistant Leuconostoc, Pediococcus, and Lactobacillus species. Antimicrob Agents Chemother 1990;34:543-9. [PubMed]
- Hamilton-Miller JM, Shah S. Vancomycin susceptibility as an aid to the identification of lactobacilli. Lett Appl Microbiol 1998;26:153-4. [PubMed]
- David V, Bozdogan B, Mainardi JL, et al. Mechanism of intrinsic resistance to vancomycin in Clostridium innocuum NCIB 10674. J Bacteriol 2004;186:3415-22. [PubMed]
- Kumar A, Augustine D, Sudhindran S, et al. Weissella confusa: a rare cause of vancomycin-resistant Gram-positive bacteraemia. J Med Microbiol 2011;60:1539-41. [PubMed]
- Geraci JE, Wilson WR. Vancomycin therapy for infective endocarditis. Rev Infect Dis 1981;3 Suppl:S250-8. [PubMed]
- Smith TL, Pearson ML, Wilcox KR, et al. Emergence of vancomycin resistance in Staphylococcus aureus. Glycopeptide-Intermediate Staphylococcus aureus Working Group. N Engl J Med 1999;340:493-501. [PubMed]
- McDonald LC, Killgore GE, Thompson A, et al. An epidemic, toxin gene-variant strain of Clostridium difficile. N Engl J Med 2005;353:2433-41. [PubMed]
- Acar J, Casewell M, Freeman J, et al. Avoparcin and virginiamycin as animal growth promoters: a plea for science in decision-making. Clin Microbiol Infect 2000;6:477-82. [PubMed]
- Bager F, Madsen M, Christensen J, et al. Avoparcin used as a growth promoter is associated with the occurrence of vancomycin-resistant Enterococcus faecium on Danish poultry and pig farms. Prev Vet Med 1997;31:95-112. [PubMed]
- Lauderdale TL, Shiau YR, Wang HY, et al. Effect of banning vancomycin analogue avoparcin on vancomycin-resistant enterococci in chicken farms in Taiwan. Environ Microbiol 2007;9:819-23. [PubMed]