Prognostic and predictive value of MET deregulation in non-small cell lung cancer
Introduction
The paradigm of non-small cell lung cancer (NSCLC) treatment has changed since the discovery of sensitizing mutations in the tyrosine kinase domain of the epidermal growth factor receptor (EGFR) and translocations involving the anaplastic lymphoma kinase (ALK), which confer exquisite sensitivity to EGFR and ALK tyrosine kinase inhibitors (TKIs), respectively (1,2). In fact, treatment of EGFR-mutant patients with gefitinib, erlotinib or afatinib and ALK-rearranged tumors with crizotinib significantly improves progression free survival (PFS) in metastatic NSCLC patients compared with standard platinum-based chemotherapy (3-6) representing successful examples of targeted therapies. However, despite these advances, the prognosis of NSCLC patients remains poor, highlighting the need of implementing prognostic and predictive factors to identify patient with high risk of relapse after surgery, to better select patients for cancer treatment and improve outcome.
MET is a receptor for the hepatocyte growth factor/scatter factor (HGF/SF) with tyrosine kinase activity that is codified by a gene located on chromosome 7q31 (7). MET is normally activated by the binding to its natural ligand, HGF, leading to receptor dimerization and trans-phosphorylation which triggers conformational changes that activate MET tyrosine kinase activity (8,9). MET signalling pathway has been found to be often deregulated in solid malignancies, including lung cancer, as a result of several mechanisms such as autocrine/paracrine stimulation, MET overexpression, genomic amplification, translocations, point mutations and alternative splicing (10). These events generally lead to increased signaling of the MET pathway, which in turn promotes tumour growth, progression and invasion (11,12), suggesting that the receptor and its ligand could represent potential prognostic markers and therapeutic targets. In addition, MET activation has been shown to be implicated in resistance to selected targeted agents, including EGFR inhibitors.
The aim of the present review is to give an overview of the relevance of MET as a prognostic marker and discusses its role as a predictor of sensitivity or resistance to anti-EGFR and MET strategies in NSCLC.
MET abnormalities in NSCLC and tools for their assessment
HGF/MET protein overexpression
HGF is the natural ligand of MET and can be secreted either by stromal, epithelial or tumor cells, resulting in increased signaling of the MET pathway by paracrine or autocrine loops. Activation of the HGF/MET axis has been observed in many types of cancers, including breast carcinomas, gliomas, osteosarcomas, melanomas and NSCLC, and it has been suggested to play a role in driving cell motility and metastasis (13-17).
The most common mechanism responsible for MET protein overexpression is transcriptional upregulation of the receptor without gene amplification, which can be triggered by a number of different events including MET crosstalk or synergies in downstream pathways. In fact, crosstalk between MET and other signaling systems, such as vascular endothelial growth factor (VEGF), EGFR, and developmental signaling pathways, like WNT-β-catenin and transforming growth factor-β-bone morphogenetic protein (TGFβ), ERBB2 or insulin-like growth factor 1 receptor (IGF1R) and others, has been reported in several models and has emerged as a major mechanism for cancer progression and resistance to treatments (10,18,19).
The most widely used tool to assess the prevalence of HGF/MET protein expression in lung cancer patients has been immunohistochemistry (IHC) performed on paraffin-embedded formalin-fixed (FFPE) samples (20-26), while fewer studies assessed circulating HGF protein levels in blood samples with ELISA assays (27-29). To date, IHC procedures and scoring methods for HGF and MET assessment have not been extensively validated, as indicated by the high degree of variability in prevalence of overexpression in unselected NSCLC series, ranging from 20% to 70% for both markers (22-26,30,31). In fact, monoclonal antibodies with different sensitivity and specificity have been used, and several different scoring systems have been investigated with a generally retrospective approach. Among several commercially available antibodies for detecting MET overexpression, the CONFIRM anti-total MET (SP44) rabbit monoclonal primary antibody (Ventana Medical Systems, Tucson, AZ, USA) is one of the most widely used, particularly in clinical trials (30,32,33). SP44 antibody is directed against a membranous and cytoplasmic epitope of MET and has been shown to have increased sensitivity when compared to other antibodies (34). More recently, a mass spectrometry approach has been proposed to accurately quantify absolute MET levels in gastroesophageal cancer FFPE tissues with high precision, although the reproducibility of the technique requires further investigation (30,33,35-37).
A key consideration when discussing HGF/MET assessment at the protein level is that the association between increased HGF levels or total MET protein expression and activation of the receptor has not been clearly demonstrated yet. In fact, in a recent study conducted in a cohort of 906 surgically resected NSCLC, IHC expression of MET and Tyr1234/1235 phospho-MET, which should indicate receptor activation, was observed in 22.2% and 5.6% of cases, respectively (30). Similarly, in a smaller Japanese study limited to resected lung adenocarcinomas a large discrepancy was observed between HGF protein expression and Tyr1234/1235 phospho-MET (57% vs. 7%) (24). While it remains unclear whether investigation of additional MET phosphorylated tyrosine residues might have increased the prevalence of cases with MET activation, these studies suggest that overexpression of HGF or MET protein does not necessarily implicate that the receptor is actually active. On the other hand, in a preclinical model of HGF-overexpressing lung adenocarcinoma cell line, total MET protein expression was reduced as a result of increased HGF-induced receptor ubiquitination and degradation (38), highlighting the notion that MET can signal regardless of its total protein levels. As of today, it remains unclear whether HGF or total MET protein overexpression represents a reliable indicator of MET activation and whether phospho-MET assessment should be preferred.
MET gene copy number (GCN)
The interest in assessing MET GCN in lung cancer has increasingly grown since 2007, when MET gene amplification was identified as a druggable mechanism of acquired resistance to EGFR TKIs in patients with EGFR-mutant NSCLC (39) and MET-amplified NSCLC cell lines exhibited exquisite sensitivity to MET inhibition (40).
The most commonly used technique to study MET gene amplification has been fluorescence in situ hybridization (FISH), a widely available tool to assess copy number changes of a selected gene in the clinical setting (i.e., HER2 gene amplification in breast and gastric cancer). Other techniques used to assess MET GCN have been reverse transcriptase polymerase chain reaction (RT PCR) and different in situ hybridization techniques, including chromogenic, silver, bright-field and dual probe in situ hybridization (CISH, SISH, BISH, DISH), which should present some advantages compared to FISH (30,33,35,37).
Several different scoring systems have been explored to evaluate MET GCN in NSCLC, including the assessment of MET/CEP7 ratio (for ISH techniques) and total MET gene copies. Depending on the goal and lung cancer populations of the several studies conducted, a number of presumably useful cut-offs have been proposed, either predefined or retrospectively identified, resulting in a great difficulty in understanding the prevalence and relevance of MET GCN gain in lung cancer (Table 1). Indeed, MET GCN can be influenced by treatments received and change over disease course. For example, several reports suggested that MET amplification is a rare event in NSCLC patients who have never received EGFR TKIs occurring only in 3-4% of cases (41,46,48), while it has been reported in up to 20% of the cases of EGFR-mutant lung adenocarcinoma with acquired resistance to EGFR TKIs (39).
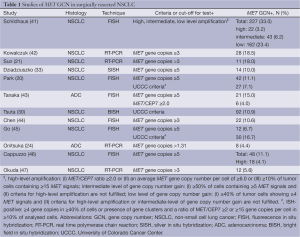
Full table
The vast majority of the studies showed no association between MET GCN and histology, gender, EGFR or KRAS gene mutations (42,46,49), while some authors observed that increased MET GCN was more frequently found in patients with advanced stage (21,44), suggesting that MET gene gain could be a late event in lung carcinogenesis. Additionally, several studies have sought to compare MET GCN assessed by different techniques and MET protein expression evaluated by IHC with conflicting results, highlighting once again the lack of standardization of the methodologies used for MET assessment (21,49,50).
MET gene mutations
MET gene activating mutations have been described in different tumor types such as hereditary and sporadic renal papillary carcinoma as germline mutations (51) and head/neck squamous cell carcinoma as somatic variants (52). Somatic mutations in NSCLC represent a rare event and have been found to occur in the juxtamembrane (53) and semaphorin extracellular domain (54), while no mutations in the tyrosine kinase domain have been reported.
Interesting data emerged from a study conducted by Krishnaswamy et al. regarding ethnic differences in the distribution of MET mutations in lung cancer patients (55). In this study, Asian, Caucasian and African American subjects with lung cancer were screened for mutations in exons coding for semaphorin, juxtamembrane and tyrosine kinase domains of MET gene. The authors found that East Asian patients had the highest frequency of mutations, the majority of which were germline. Of note MET mutations were associated with squamous cell histology and had the highest prevalence in male smokers, suggesting a possible relationship between tobacco smoking exposure, MET mutations and potential risk of developing lung cancer.
Due to the lack of a comprehensive functional characterization of MET mutations, with the exception of a few variants (51) and in absence of data supporting their prognostic or predictive role, MET mutations will not be further discussed in the present review.
Prognostic value of HGF/MET
The impact of the activation of the MET pathway on survival of lung cancer patients has been mostly studied in terms of MET GCN and HGF/MET overexpression in surgically resected patients, while the prognostic impact of HGF/MET aberrations in an unselected metastatic setting remains largely unexplored.
MET GCN
The possible prognostic value of increased MET GCN was suggested for the first time by Okuda et al. (47) in a Japanese cohort of radically resected NSCLC. MET gene gain was analyzed by quantitative real time PCR in 213 surgical samples of NSCLC. Increased MET GCN (more than 3 copies per cell) was identified in 12 patients, all of whom were male and smokers (5.6%) and resulted in a statistically significant worse prognosis in terms of overall survival (OS; P=0.041) although this finding was not confirmed by multivariate analysis.
Subsequently, Cappuzzo et al. conducted a larger retrospective analysis (46) evaluating the prognostic effect of MET/EGFR gene status by FISH in 447 cases of surgical NSCLC. FISH analysis was positive (≥5 copies per cell) for MET in 48 cases (11.1%) and associated with advanced stage and grade 3. MET FISH positive patients had a significantly shorter survival in comparison with MET FISH negative patients (25.8 vs. 47.5 months; P=0.005). This observation was confirmed in the multivariable model, providing the first evidence that MET gene gain assessed with the abovementioned FISH criteria could represent an independent unfavorable prognostic factor, regardless of clinico-pathological characteristics. A further interesting finding of this study was the statistically significant association described between increased GCN of MET and EGFR, both located on chromosome 7, suggesting that this chromosomic area is generally interested by broad genomic gain events or polisomy rather than focal amplifications.
Similar data have been reported in later studies employing different technologies for MET assessment (20,30,44,45,49), although conflicting results regarding the impact of histology on the prognostic relevance of MET GCN have emerged. In fact, while some authors reported that the negative survival impact for MET gene gain was limited to patients with squamous cell cancer (45), other investigators observed a prognostic role for MET GCN only in lung adenocarcinoma (20,43).
A recent meta-analysis by Dimou et al. (56) combined results from nine studies that investigated the association of MET GCN with survival in NSCLC patients who received surgery. When all studies were analyzed in a random-effect model, the net HR (HR, 1.78) indicated a worse OS for patients with higher MET GCN. When grouped according to histology only studies that had at least 50% of adenocarcinoma had a significantly poor prognosis, suggesting that the prognostic impact of MET GCN may be adenocarcinoma histology specific. Similarly, a meta-analysis by Guo et al. including 18 retrospective trials, confirmed the independent negative prognostic impact for increased MET GCN in surgically resected NSCLC, with a more pronounced effect in lung adenocarcinoma as compared with squamous cell cancer (57).
Finally, it should be noted that from a methodological point of view the majority of the suggested cut-offs for increased MET GCN have been functional to the identification of groups of patients with a different prognosis but do not necessarily contribute to understand which tumors are MET-dependent.
MET protein expression
MET protein overexpression assessed by IHC has been shown to retain a negative prognostic value in surgically resected NSCLC cohorts (20,21,58). Park et al. observed a MET IHC-positive rate of 13.7% in a cohort of 380 Korean NSCLC patients treated with surgery. MET-overexpressing cases had a statistically significantly shorter OS than patients with no MET protein expression (P=0.01), and this finding was confirmed by multivariate analysis (HR, 1.618; 95% confidence interval, 1.066-2.456; P=0.024).
Other studies conducted in unselected NSCLC or specifically in lung adenocarcinoma produced non-conclusive results. Tsuta et al. assessed MET and phospho-MET protein expression by IHC, as well as MET GCN by BISH, in tissue microarrays from a large cohort of resected Japanese NSCLCs (n=906) (30). MET/phospho-MET expression had no impact on survival in the whole cohort nor when grouping patients according to histology (Table 2).

Full table
The discrepancy between the findings of the several studies performed in this setting led to a meta-analysis (57) with the aim of better understanding the prognostic impact of MET protein expression in resected patients. Importantly, in the multivariate model which included 8 retrospective studies, MET expression was found to independently double the risk of mortality, even when excluding those studies responsible for heterogeneity (HR, 2.00, P<0.001).
Circulating and intratumoral HGF protein levels
Japanese investigators have explored the prognostic impact of a number of circulating factors, including HGF, in a cohort of 109 patients undergoing preoperative chemotherapy and surgery (27). When using the median serum HGF value measured with ELISA as a cut off, patients with low HGF levels had a significantly better OS than those with high HGF levels (P=0.019), and this finding was confirmed by multivariate analysis in the subset of stage III patients. Similar studies have been conducted in smaller cohorts of surgically resected patients with contrasting results (59,60), likely due to the small sample size.
Some efforts have been carried out to test whether intratumoral HGF protein expression could affect survival in surgically resected NSCLC patients. The first evidence supporting an independent negative prognostic role for HGF overexpression at the tumor level came from a small study conducted in 56 resectable NSCLC patients (61) where HGF protein was quantified by Western Blot. When using median HGF levels as a cutoff, patients with increased HGF protein expression had a shorter OS than patients with HGF below the median value (P=0.03), and this observation was confirmed in the multivariate analysis (P=0.0001). Other studies have sought to explore the survival impact of intratumoral protein levels of HGF with IHC (24,62,63). One of the largest studies was performed in 183 resected lung adenocarcinomas and detected HGF overexpression in 57% of cases. HGF-positive status was associated with MET phosphorylation (Tyr 1234/1235) and predicted worse OS in univariate and multivariate analysis.
Predictive value of MET
The predictive role of MET activation has been explored in different settings, including NSCLC patients treated with EGFR TKIs and those receiving anti-MET based strategies.
EGFR TKIs
In preclinical studies conducted in lung adenocarcinoma cells harboring EGFR mutations high levels of HGF immunoreactivity were detected as a potential mechanisms of primary resistance mediated by the MET/PI3K signaling axis (64,65). This hypothesis has been confirmed in a later study by the same group which detected high levels of HGF expression in 61% non-responders patients with intrinsic or acquired resistance to EGFR-TKIs, suggesting a role of HGF in contributing to EGFR TKIs resistance (66,67). In fact, as shown by Turke et al., high levels of HGF could mediate the EGFR-TKI resistance either by stimulating MET amplification or autocrine HGF production (68).
Engelman et al. for the first time demonstrated that MET amplification represents one of the main mechanisms of EGFR TKI acquired resistance by driving the ERBB3-dependent activation of PI3K occurring under drug pressure (39). However, primary resistance to EGFR TKI in patients harboring MET gene copy gain before treatment is a rare event (48). On the other hand, Benedettini et al. reported that MET protein expression and phosphorylation evaluated before EGFR TKI treatment correlated with shorter time to progression suggesting a potential role of MET activation in de novo resistance to EGFR inhibitors (69). These results are consistent with the data from Zucali et al. showing that MET protein expression was significantly associated with primary resistance to gefitinib in advanced NSCLC. Moreover in order to test the role of MET in resistance mechanisms, they evaluated the combination of the MET antibody DN-30 with gefitinb in a panel of NSCLC cell lines showing that the addition of DN-30 to gefitinb enhance the growth inhibition of cell lines with positive MET expression (70).
More recently preclinical data have shown that MET receptor can induce gefitinib resistance through regulation of expression of specific miRNAs (71) suggesting that modulation of specific miRNAs may provide a new therapeutic approach to lung cancer treatment.
Anti-MET strategies
Since preclinical evidence has shown that cell lines with MET overexpression or MET gene amplification made tumor cells addicted to this oncogene (72-74), several anti-MET strategies have been explored in the clinical setting. Inhibition of MET can be achieved with monoclonal antibodies targeting the receptor or its ligand or small TKIs exerting their activity at the intracellular level by inhibition of the kinase domain of the receptor.
HGF/MET monoclonal antibodies
A number of monoclonal antibodies aimed at disrupting MET signaling have been evaluated in the clinical setting, including agents that bind to HGF preventing its interaction with MET (rilotumumab, ficlatuzumab), and molecules that bind to the extracellular domain of MET (onartuzumab) (75-77).
Rilotumumab (AMG 102; Amgen, Thousand Oaks, CA, USA) has been evaluated in a phase I study in unselected solid tumors in combination with VEGF pathway inhibitors bevacizumab or motesanib and showed acceptable toxicity in patients receiving rilotumumab plus bevacizumab with mild fatigue, nausea, constipation and peripheral edema (78). An early phase II study of rilotumumab failed to demonstrate a benefit in patients with glioblastoma (79). To date, a phase I/II study evaluating a combination of rilotumumab and erlotinib in unselected pretreated metastatic NSCLC is ongoing (ClinTrial.gov, NCT01233687).
Fliclatuzumab (AV-299; AVEO, Cambridge, MA, USA) has been evaluated in some phase I trials alone or in combination with EGFR inhibitors erlotinib or gefitinib in patients with solid tumors, including NSCLC, with a favorable toxicity profile (80). More recently, Mok et al. presented the results of a randomized phase II trial comparing gefitinib alone versus gefitinib plus fliclatuzumab in Asian patients with lung adenocarcinoma with clinical predictors of presence of EGFR activating mutations. The study, whose primary end point was to compare overall response rate between treatment arms, was conceived due to the preclinical evidence that HGF upregulation can be responsible for acquired resistance to EGFR TKIs in NSCLC with EGFR mutations, as previously discussed (64,66,68). In the intent to treat (ITT) population there was neither statistical difference in response rate (40% for gefitinib arm versus 43% for combination arm) nor PFS (4.7 months in gefitinib arm vs. 5.6 months in the combination arm). However, in subgroup analyses it emerged that patients with EGFR mutations and low MET expression benefited more from the combination treatment arm in terms of PFS suggesting that inhibition of HGF/MET could delay resistance to EGFR TKIs in EGFR-mutant patients (80,81). To better clarify the activity of a two-drug approach in this setting phase II trials evaluating fliclatuzumab in combination with erlotinib in EGFR mutant patients are ongoing (clinicaltrials.gov, NCT 02318368).
Onartuzumab (MetMab; Genentech-Roche) is a monoclonal antibody directed against the semaphorin domain of MET thus blocking the ligand-induced dimerization and activation of tyrosine kinase activity of the receptor (82). Efficacy data from onartuzumab derived from a global randomized double-blind phase II trial comparing erlotinib plus onartuzumab or placebo as second or third line treatment in advanced NSCLC patients. The co-primary end points were PFS in the whole population and in MET expression positive patients evaluated by IHC. Importantly, MET status was determined after random assignment and before unblinding and IHC scoring were predefined as follows: 3+ (≥50% of tumor cells staining with strong intensity); 2+ (≥50% of tumor cells with moderate or higher staining but <−50% with strong intensity); 1+ (≥50% of tumor cells with weak or higher staining but <-50% with moderate or higher intensity); or 0 (no staining or <50% of tumor cells with any intensity). Patients with 2+ or 3+ score were considered MET-positive. Patients were stratified according to smoking history and histology. In the ITT population (n=137) there was no difference in terms of PFS between treatment arms (median 2.9 months for placebo plus erlotinib vs. 2.2 months for onartuzumab plus erlotinib; HR, 1.09; P=0.69) or OS (median 7.4 months for placebo plus erlotinib versus 8.9 months in the experimental arm; HR, 0.80; P=0.34). However, when considering only the 66 MET-positive patients (52%) the experimental arm resulted in both clinically and statistically significant improved PFS (HR, 0.53; P=0.04) and OS (HR, 0.37; P=0.002) which translated in twofold reduction in the risk of disease progression and threefold reduction in the risk of death compared with erlotinib alone (32,83). Noteworthy, the benefit in MET-positive patients was independent of FISH analysis for MET GCN although the small number of patients (n=19) with MET gene gain (≥5 copies) precluded any conclusion (83). Interestingly, the outcome was worse in the MET-negative population treated with onartuzumab plus erlotinib in terms of PFS as well as OS (HR, 2.01 and 3.02 respectively) (32) highlighting the relevance of proper patient selection for such combination therapy. Additional biomarker analyses failed to show any role for MET, EGFR, amphiregulin, epiregulin, or HGF mRNA expression to predict a benefit from onartuzumab plus erlotinib, suggesting that MET IHC could be the best predictor of onartuzumab activity (83).
On the wave of the promising results of the phase II trial, a global double-blind phase III study comparing onartuzumab plus erlotinib vs. placebo plus erlotinib in pretreated MET positive (2+/3+) metastatic NSCLC patients (MetLung study) was performed (84). The primary end point was OS. A total of 480 patients worldwide have been enrolled, but unfortunately the trial closed prematurely after an interim analysis showing no difference in OS (6.8 vs. 9.1 months for onartuzumab + erlotinib vs. erlotinib; HR, 1.27; P=0.07) and PFS (2.7 vs. 2.6 months for the experimental vs. control arm; HR, 0.99, P=0.92). Surprisingly, in the subgroup analysis, patients harboring EGFR mutations allocated in the experimental arm seemed to have a detrimental effect from the combination treatment. Such disappointing results could be related to the low predictive value of MET protein expression as a tool to detect MET activation and to select patients for anti-MET strategies. In fact, we still do not know whether MET overexpression, which can be triggered from different biological events, reflects tumor cells dependency on the MET pathway. Additionally, it remains unclear whether onartuzumab is active against tumors with MET-amplification, which has been shown to represent a model of oncogene addiction in vitro (39,40,73). Indeed, as recently showed by Arriola et al., MET expression assessed by IHC with different scoring criteria, including the onartuzumab scoring system, did not correlate with MET gene amplification in small NSCLC biopsies (50), highlighting the challenges in the identification of MET-driven tumors.
MET TKIs
Several small molecules directed against the intracellular tyrosine kinase domain of MET have been characterized preclinically and some of them have reached the clinical trial setting with variable results. As compared with monoclonal antibodies these agents are administered orally and act at the intracellular level, regardless of ligand-receptor interactions.
Tivantinib (ARQ 197; ArQule Woburn, MA, USA; Daiichi Sankyo, Tokyo, Japan) is a non-adenosine-triphosphate competitive small molecule selective for MET receptor. The compound has been shown to act as a potent MET inhibitor but at the same time preclinical data suggested that tivantinib exerts citotoxic activity independently of MET status through disruption of microtubules polymerization (85-87).
Due to the role of MET in contributing to intrinsic and acquired resistance to EGFR TKIs, a phase II randomized, double blind, placebo-controlled trial of erlotinib in combination with tivantinib or placebo in 167 pretreated, TKIs naïve, metastatic NSCLC patients was performed (88). The primary end point was PFS in the ITT population. Patients were unselected for molecular characteristic, but a biomarker analysis was performed after study entry. In the ITT population there was a modest trend for improved PFS in favor of the experimental arm, although the difference did not reach statistical significance (3.8 vs. 2.3 months for the combination vs. control arm, respectively; HR, 0.81; P=0.24). The PFS advantage became significant after adjustment for known prognostic factors (HR, 0.68, P=0.04), but subgroup analyses revealed that only nonsquamous patients seemed to benefit from the combination arm, both in terms of PFS (HR, 0.61, P=0.04) and OS (HR, 0.58, P=0.04). Of note, in the preplanned exploratory analysis of PFS and OS according to molecular characteristics it emerged that patients with increased MET GCN had a trend toward a benefit for the tivantinib plus erlotinib arm and the benefit grew in magnitude as the copy number cutoff rose, although not statistically significant. Importantly, in patients with low MET GCN (<2 or <3) there was no difference in efficacy between the two treatment arms (88). Additionally, despite small patient numbers, a significant improvement in PFS (HR, 0.18, P<0.01) was observed in KRAS-mutant patients, while a trend for longer PFS was observed in patients with wild type EGFR (HR, 0.70; P=0.12).
Based on these results the confirmatory phase III MARQUEE trial (Met inhibitor ARQ 197 plus erlotinib vs. erlotinib plus placebo) in previously treated, TKIs naïve, metastatic non-squamous NSCLC patients, was conducted and enrolled 998 unselected patients stratified according to EGFR and KRAS gene status (89). The primary end point was OS but unfortunately the trial was halted after an interim analysis showing that the study would not meet its primary end point of improved OS (HR, 0.98). Of note, the interim analysis did show a statistically significant improvement in PFS but this benefit did not translate into OS prolongation. Although the trial was negative it is noteworthy that in approximately 40% of enrolled patients for which tumor tissue was available MET was evaluated by IHC showing a substantial improvement in both PFS and OS in MET high (2+/3+ in >50% of cells) patients treated with tivantinib plus erlotinib. The final results of biomarker analysis will further clarify whether the combination of erlotinib and tivantinib can prove active in molecularly selected NSCLC patients.
Crizotinib (PF 02341006, Xalkori®) is a potent ALK and ROS1 inhibitor, approved by FDA in 2011 for patients harboring ALK translocations, that was initially developed as a MET inhibitor. In a preclinical study crizotinib showed a marked antiproliferative effect in MET-amplified cell (90), confirming previous evidence that MET amplification results in constitutive activation of the receptor and represents a model of oncogene addiction. Ou et al. reported a case of a patient with de novo high level MET amplification assessed by FISH (MET/CEP7 ratio >5.0) but without ALK translocation who achieved a rapid and durable response to crizotinib (91), confirming that the drug is a potent MET inhibitor and that MET amplification could be a good predictor of response.
More recently, the activity and safety of crizotinib in MET-amplified metastatic NSCLC patients has been investigated in a phase I study (92). Patients were selected according to MET amplification assessed by FISH and divided into three categories according to MET/CEP7 ratio (low: 1.8-2.2; intermediate 2.2-5.0; high: ≥5.0). The preliminary results in 12 patients showed remarkable tumor shrinkage in the group with intermediate and high levels of MET-amplification, with response rates of 17% and 67%, respectively. Interestingly, the subjects who achieved a greater response were mainly smokers and, although the small number of patients preclude any conclusion, this is consistent with several reports (45,47) suggesting a possible relationship between MET gene amplification and squamous cell carcinoma’s oncogenesis. Exploratory analyses to identify the optimal MET/CEP7 ratio to predict clinical benefit from crizotinib are ongoing.
Cabozantinib (XL184/BMS-907351; Exelixis South San Francisco, CA, USA/Bristol-Meyers Squibb) is an oral multikinase inhibitor targeting MET, VEGFR, FLT3, RET and KIT that has recently received FDA approval for metastatic medullary thyroid cancer (93). Cabozantinib has been evaluated in a phase I/II study in combination with erlotinib in NSCLC patients with acquired resistance to erlotinib. The study enrolled 54 patients showing encouraging clinical activity of cabozantinib plus erlotinib in erlotinib-pretreated population including patients with MET amplification and T790M EGFR mutation (94). Clinical activity of cabozantinib was further investigated in a larger phase II study involving several solid tumors where MET signaling plays an important role, including NSCLC (n=60), where partial responses were observed in six patients (13%). Interestingly, some of the responders harbored driver mutations (EGFR or KRAS mutations) at baseline while non-responders did not (54,95).
Conclusions
The HGF/MET pathway represents an extremely appealing therapeutic target in NSCLC, although clinical trials with anti-HGF/MET strategies in patients with advanced disease have produced mixed results. While it is becoming increasingly clear that MET gene amplification confers dependency on MET for survival of tumor cells and is susceptible of drug inhibition with MET TKIs in the clinical setting, the role of MET IHC to select patients for anti-MET agents remains unclear.
In the setting of surgically resected NSCLC, increased MET GCN or protein levels of HGF/MET could help to identify subjects with a higher risk of death, highlighting the need for effective adjuvant approaches for these patients.
Acknowledgements
Disclosure: The authors declare no conflict of interest.
References
- Lynch TJ, Bell DW, Sordella R, et al. Activating mutations in the epidermal growth factor receptor underlying responsiveness of non-small-cell lung cancer to gefitinib. N Engl J Med 2004;350:2129-39. [PubMed]
- Soda M, Choi YL, Enomoto M, et al. Identification of the transforming EML4-ALK fusion gene in non-small-cell lung cancer. Nature 2007;448:561-6. [PubMed]
- Mok TS, Wu YL, Thongprasert S, et al. Gefitinib or carboplatin-paclitaxel in pulmonary adenocarcinoma. N Engl J Med 2009;361:947-57. [PubMed]
- Shaw AT, Kim DW, Nakagawa K, et al. Crizotinib versus chemotherapy in advanced ALK-positive lung cancer. N Engl J Med 2013;368:2385-94. [PubMed]
- Rosell R, Carcereny E, Gervais R, et al. Erlotinib versus standard chemotherapy as first-line treatment for European patients with advanced EGFR mutation-positive non-small-cell lung cancer (EURTAC): A multicentre, open-label, randomised phase 3 trial. Lancet Oncol 2012;13:239-46. [PubMed]
- Yang JC, Wu YL, Schuler M, et al. Afatinib versus cisplatin-based chemotherapy for EGFR mutation-positive lung adenocarcinoma (LUX-Lung 3 and LUX-Lung 6): analysis of overall survival data from two randomised, phase 3 trials. Lancet Oncol 2015;16:141-51. [PubMed]
- Weir BA, Woo MS, Getz G, et al. Characterizing the cancer genome in lung adenocarcinoma. Nature 2007;450:893-8. [PubMed]
- Rong S, Segal S, Anver M, et al. Invasiveness and metastasis of NIH 3T3 cells induced by Met-hepatocyte growth factor/scatter factor autocrine stimulation. Proc Natl Acad Sci U S A 1994;91:4731-5. [PubMed]
- Toschi L, Jänne PA. Single-agent and combination therapeutic strategies to inhibit hepatocyte growth factor/MET signaling in cancer. Clin Cancer Res 2008;14:5941-6. [PubMed]
- Gherardi E, Birchmeier W, Birchmeier C, et al. Targeting MET in cancer: rationale and progress. Nat Rev Cancer 2012;12:89-103. [PubMed]
- Birchmeier C, Birchmeier W, Gherardi E, et al. Met, metastasis, motility and more. Nat Rev Mol Cell Biol 2003;4:915-25. [PubMed]
- Comoglio PM, Giordano S, Trusolino L. Drug development of MET inhibitors: targeting oncogene addiction and expedience. Nat Rev Drug Discov 2008;7:504-16. [PubMed]
- Trusolino L, Bertotti A, Comoglio PM. MET signalling: principles and functions in development, organ regeneration and cancer. Nat Rev Mol Cell Biol 2010;11:834-48. [PubMed]
- Tuck AB, Park M, Sterns EE, et al. Coexpression of hepatocyte growth factor and receptor (Met) in human breast carcinoma. Am J Pathol 1996;148:225-32. [PubMed]
- Koochekpour S, Jeffers M, Rulong S, et al. Met and hepatocyte growth factor/scatter factor expression in human gliomas. Cancer Res 1997;57:5391-8. [PubMed]
- Li G, Schaider H, Satyamoorthy K, et al. Downregulation of E-cadherin and Desmoglein 1 by autocrine hepatocyte growth factor during melanoma development. Oncogene 2001;20:8125-35. [PubMed]
- Ferracini R, Angelini P, Cagliero E, et al. MET oncogene aberrant expression in canine osteosarcoma. J Orthop Res 2000;18:253-6. [PubMed]
- Bauer TW, Somcio RJ, Fan F, et al. Regulatory role of c-Met in insulin-like growth factor-I receptor-mediated migration and invasion of human pancreatic carcinoma cells. Mol Cancer Ther 2006;5:1676-82. [PubMed]
- Peters S, Adjei AA. MET: a promising anticancer therapeutic target. Nature Reviews Clinical Oncology 2012;9:314-26. [PubMed]
- Park S, Choi YL, Sung CO, et al. High MET copy number and MET overexpression: poor outcome in non-small cell lung cancer patients. Histol Histopathol 2012;27:197-207. [PubMed]
- Sun W, Song L, Ai T, et al. Prognostic value of MET, cyclin D1 and MET gene copy number in non-small cell lung cancer. J Biomed Res 2013;27:220-30. [PubMed]
- Ma PC, Tretiakova MS, MacKinnon AC, et al. Expression and mutational analysis of MET in human solid cancers. Genes Chromosomes Cancer 2008;47:1025-37. [PubMed]
- Tsao MS, Zhu H, Giaid A, et al. Hepatocyte growth factor/scatter factor is an autocrine factor for human normal bronchial epithelial and lung carcinoma cells. Cell Growth Differ 1993;4:571-9. [PubMed]
- Onitsuka T, Uramoto H, Ono K, et al. Comprehensive molecular analyses of lung adenocarcinoma with regard to the epidermal growth factor receptor, K-ras, MET, and hepatocyte growth factor status. J Thorac Oncol 2010;5:591-6. [PubMed]
- Olivero M, Valente G, Bardelli A, et al. Novel mutation in the ATP-binding site of the MET oncogene tyrosine kinase in a HPRCC family. Int J Cancer 1999;82:640-3. [PubMed]
- Tsao MS, Yang Y, Marcus A, et al. Hepatocyte growth factor is predominantly expressed by the carcinoma cells in non-small-cell lung cancer. Hum Pathol 2001;32:57-65. [PubMed]
- Ujiie H, Tomida M, Akiyama H, et al. Serum hepatocyte growth factor and interleukin-6 are effective prognostic markers for non-small cell lung cancer. Anticancer Res 2012;32:3251-8. [PubMed]
- Masago K, Togashi Y, Fujita S, et al. Clinical significance of serum hepatocyte growth factor and epidermal growth factor gene somatic mutations in patients with non-squamous non-small cell lung cancer receiving gefitinib or erlotinib. Med Oncol 2012;29:1614-21. [PubMed]
- Kasahara K, Arao T, Sakai K, et al. Impact of serum hepatocyte growth factor on treatment response to epidermal growth factor receptor tyrosine kinase inhibitors in patients with non-small cell lung adenocarcinoma. Clin Cancer Res 2010;16:4616-24. [PubMed]
- Tsuta K, Kozu Y, Mimae T, et al. c-MET/phospho-MET protein expression and MET gene copy number in non-small cell lung carcinomas. J Thorac Oncol 2012;7:331-9. [PubMed]
- Koeppen H, Rost S, Yauch RL. Developing biomarkers to predict benefit from HGF/MET pathway inhibitors. Journal of Pathology 2014;232:210-8. [PubMed]
- Spigel DR, Ervin TJ, Ramlau RA, et al. Randomized phase II trial of Onartuzumab in combination with erlotinib in patients with advanced non-small-cell lung cancer. J Clin Oncol 2013;31:4105-14. [PubMed]
- Dziadziuszko R, Wynes MW, Singh S, et al. Correlation between MET gene copy number by silver in situ hybridization and protein expression by immunohistochemistry in non-small cell lung cancer. J Thorac Oncol 2012;7:340-7. [PubMed]
- Ha SY, Lee J, Kang SY, et al. MET overexpression assessed by new interpretation method predicts gene amplification and poor survival in advanced gastric carcinomas. Mod Pathol 2013;26:1632-41. [PubMed]
- Summersgill B, Clark J, Shipley J. Fluorescence and chromogenic in situ hybridization to detect genetic aberrations in formalin-fixed paraffin embedded material, including tissue microarrays. Nat Protoc 2008;3:220-34. [PubMed]
- Catenacci DV, Liao WL, Thyparambil S, et al. Absolute quantitation of Met using mass spectrometry for clinical application: assay precision, stability, and correlation with MET gene amplification in FFPE tumor tissue. PLoS One 2014;9:e100586. [PubMed]
- Feng Y, Minca EC, Lanigan C, et al. High MET receptor expression but not gene amplification in ALK 2p23 rearrangement positive non-small-cell lung cancer. J Thorac Oncol 2014;9:646-53. [PubMed]
- Sano Y, Hashimoto E, Nakatani N, et al. Combining Onartuzumab with Erlotinib Inhibits Growth of Non-Small Cell Lung Cancer with Activating EGFR Mutations and HGF Overexpression. Mol Cancer Ther 2015;14:533-41. [PubMed]
- Engelman JA, Zejnullahu K, Mitsudomi T, et al. MET amplification leads to gefitinib resistance in lung cancer by activating ERBB3 signaling. Science 2007;316:1039-43. [PubMed]
- Lutterbach B, Zeng Q, Davis LJ, et al. Lung cancer cell lines harboring MET gene amplification are dependent on Met for growth and survival. Cancer Res 2007;67:2081-8. [PubMed]
- Schildhaus HU, Schultheis AM, Rüschoff J, et al. MET Amplification Status in Therapy-Naïve Adeno- and Squamous Cell Carcinomas of the Lung. Clin Cancer Res 2015;21:907-15. [PubMed]
- Kowalczuk O, Kozlowski M, Niklinska W, et al. Increased MET Gene Copy Number but Not mRNA Level Predicts Postoperative Recurrence in Patients with Non-Small Cell Lung Cancer. Transl Oncol 2014;7:605-12. [PubMed]
- Tanaka A, Sueoka-Aragane N, Nakamura T, et al. Co-existence of positive MET FISH status with EGFR mutations signifies poor prognosis in lung adenocarcinoma patients. Lung Cancer 2012;75:89-94. [PubMed]
- Chen YT, Chang JW, Liu HP, et al. Clinical implications of high MET gene dosage in non-small cell lung cancer patients without previous tyrosine kinase inhibitor treatment. J Thorac Oncol 2011;6:2027-35. [PubMed]
- Go H, Jeon YK, Park HJ, et al. High MET gene copy number leads to shorter survival in patients with non-small cell lung cancer. J Thorac Oncol 2010;5:305-13. [PubMed]
- Cappuzzo F, Marchetti A, Skokan M, et al. Increased MET gene copy number negatively affects survival of surgically resected non-small-cell lung cancer patients. J Clin Oncol 2009;27:1667-74. [PubMed]
- Okuda K, Sasaki H, Yukiue H, et al. Met gene copy number predicts the prognosis for completely resected non-small cell lung cancer. Cancer Sci 2008;99:2280-5. [PubMed]
- Cappuzzo F, Jänne PA, Skokan M, et al. MET increased gene copy number and primary resistance to gefitinib therapy in non-small-cell lung cancer patients. Ann Oncol 2009;20:298-304. [PubMed]
- Jin Y, Sun PL, Kim H, et al. MET gene copy number gain is an independent poor prognostic marker in Korean stage I lung adenocarcinomas. Ann Surg Oncol 2014;21:621-8. [PubMed]
- Arriola E, Casadevall D, Gimeno J, et al. Met heterogeneity evaluation by immunohistochemistry (IHC) and fluorescence in situ hybridization (FISH) in nonsquamous non-small cell lung cancer (nsNSCLC). J Clin Oncol 2014;32:abstr 11005.
- Schmidt L, Duh FM, Chen F, et al. Germline and somatic mutations in the tyrosine kinase domain of the MET proto-oncogene in papillary renal carcinomas. Nat Genet 1997;16:68-73. [PubMed]
- Aebersold DM, Landt O, Berthou S, et al. Prevalence and clinical impact of Met Y1253D-activating point mutation in radiotherapy-treated squamous cell cancer of the oropharynx. Oncogene 2003;22:8519-23. [PubMed]
- Kong-Beltran M, Seshagiri S, Zha J, et al. Somatic mutations lead to an oncogenic deletion of Met in lung cancer. Cancer Res 2006;66:283-9. [PubMed]
- Sadiq AA, Salgia R. MET as a possible target for non-small-cell lung cancer. J Clin Oncol 2013;31:1089-96. [PubMed]
- Krishnaswamy S, Kanteti R, Duke-Cohan JS, et al. Ethnic Differences and Functional Analysis of MET Mutations in Lung Cancer. Clin Cancer Res 2009;15:5714-23. [PubMed]
- Dimou A, Non L, Chae YK, et al. MET gene copy number predicts worse overall survival in patients with non-small cell lung cancer (NSCLC); a systematic review and meta-analysis. PLoS One 2014;9:e107677. [PubMed]
- Guo B, Cen H, Tan X, et al. Prognostic value of MET gene copy number and protein expression in patients with surgically resected non-small cell lung cancer: a meta-analysis of published literatures. PLoS One 2014;9:e99399. [PubMed]
- Galleges Ruiz MI, Floor K, et al. Combined assessment of EGFR pathway-related molecular markers and prognosis of NSCLC patients. Br J Cancer 2009;100:145-52. [PubMed]
- Hosoda H, Izumi H, Tukada Y, et al. Plasma hepatocyte growth factor elevation may be associated with early metastatic disease in primary lung cancer patients. Ann Thorac Cardiovasc Surg 2012;18:1-7. [PubMed]
- Fang MY, Wang SY, Zheng YB, et al. Prognostic and predictive significance of plasma hepatocyte growth factor and carcinoembryonic antigen in non-small lung cancer after surgery. Eur Rev Med Pharmacol Sci 2014;18:398-403. [PubMed]
- Siegfried JM, Weissfeld LA, Singh-Kaw P, et al. Association of immunoreactive hepatocyte growth factor with poor survival in resectable non-small cell lung cancer. Cancer Res 1997;57:433-9. [PubMed]
- Takanami I, Tanana F, Hashizume T, et al. Hepatocyte growth factor and c-Met/hepatocyte growth factor receptor in pulmonary adenocarcinomas: an evaluation of their expression as prognostic markers. Oncology 1996;53:392-7. [PubMed]
- Masuya D, Huang C, Liu D, et al. The tumour-stromal interaction between intratumoral c-Met and stromal hepatocyte growth factor associated with tumour growth and prognosis in non-small-cell lung cancer patients. Br J Cancer 2004;90:1555-62. [PubMed]
- Yano S, Wang W, Li Q, et al. Hepatocyte growth factor induces gefitinib resistance of lung adenocarcinoma with epidermal growth factor receptor-activating mutations. Cancer Res 2008;68:9479-87. [PubMed]
- Okamoto W, Okamoto I, Tanaka K, et al. TAK-701, a humanized monoclonal antibody to hepatocyte growth factor, reverses gefitinib resistance induced by tumor-derived HGF in non-small cell lung cancer with an EGFR mutation. Mol Cancer Ther 2010;9:2785-92. [PubMed]
- Yano S, Yamada T, Takeuchi S, et al. Hepatocyte growth factor expression in EGFR mutant lung cancer with intrinsic and acquired resistance to tyrosine kinase inhibitors in a Japanese cohort. J Thorac Oncol 2011;6:2011-7. [PubMed]
- Han JY, Kim JY, Lee SH, et al. Association between plasma hepatocyte growth factor and gefitinib resistance in patients with advanced non-small cell lung cancer. Lung Cancer 2011;74:293-9. [PubMed]
- Turke AB, Zejnullahu K, Wu YL, et al. Preexistence and clonal selection of MET amplification in EGFR mutant NSCLC. Cancer Cell 2010;17:77-88. [PubMed]
- Benedettini E, Sholl LM, Peyton M, et al. Met activation in non-small cell lung cancer is associated with de novo resistance to EGFR inhibitors and the development of brain metastasis. Am J Pathol 2010;177:415-23. [PubMed]
- Zucali PA, Ruiz MG, Giovannetti E, et al. Role of cMET expression in non-small-cell lung cancer patients treated with EGFR tyrosine kinase inhibitors. Ann Oncol 2008;19:1605-12. [PubMed]
- Garofalo M, Romano G, Di Leva G, et al. EGFR and MET receptor tyrosine kinase-altered microRNA expression induces tumorigenesis and gefitinib resistance in lung cancers. Nat Med 2012;18:74-82. [PubMed]
- Lennerz JK, Kwak EL, Ackerman A, et al. MET amplification identifies a small and aggressive subgroup of esophagogastric adenocarcinoma with evidence of responsiveness to crizotinib. J Clin Oncol 2011;29:4803-10. [PubMed]
- Smolen GA, Sordella R, Muir B, et al. Amplification of MET may identify a subset of cancers with extreme sensitivity to the selective tyrosine kinase inhibitor PHA-665752. Proc Natl Acad Sci U S A 2006;103:2316-21. [PubMed]
- Corso S, Migliore C, Ghiso E, et al. Silencing the MET oncogene leads to regression of experimental tumors and metastases. Oncogene 2008;27:684-93. [PubMed]
- Sadiq AA, Salgia R. MET as a possible target for non-small-cell lung cancer. J Clin Oncol 2013;31:1089-96. [PubMed]
- Sierra JR, Tsao MS. c-MET as a potential therapeutic target and biomarker in cancer. Ther Adv Med Oncol 2011;3:S21-35. [PubMed]
- Smyth EC, Sclafani F, Cunningham D. Emerging molecular targets in oncology: clinical potential of MET/hepatocyte growth-factor inhibitors. Onco Targets Ther 2014;7:1001-14. [PubMed]
- Rosen PJ, Sweeney CJ, Park DJ, et al. A phase Ib study of AMG 102 in combination with bevacizumab or motesanib in patients with advanced solid tumors. Clin Cancer Res 2010;16:2677-87. [PubMed]
- Wen PY, Schiff D, Cloughesy TF, et al. A phase II study evaluating the efficacy and safety of AMG 102 (rilotumumab) in patients with recurrent glioblastoma. Neuro Oncol 2011;13:437-46. [PubMed]
- D’Arcangelo M, Cappuzzo F. Focus on the potential role of ficlatuzumab in the treatment of non-small cell lung cancer. Biologics 2013;7:61-8. [PubMed]
- Mok TS, Park K, Geater SL, et al. A randomized phase (PH) 2 study with exploratory biomarker analysis of ficlatuzumab (F) a humanized hepatocyte growth factor (HGF) inhibitory mAb in combination with gefitinib (G) versus G in Asian patients (pts) with lung Adenocarcinoma (LA). Ann Oncol 2012;23:ix391.
- Martens T, Schmidt NO, Eckerich C, et al. A novel one-armed anti-c-Met antibody inhibits glioblastoma growth in vivo. Clin Cancer Res 2006;12:6144-52. [PubMed]
- Koeppen H, Yu W, Zha J, et al. Biomarker analyses from a placebo-controlled phase II study evaluating erlotinib±onartuzumab in advanced non-small cell lung cancer: MET expression levels are predictive of patient benefit. Clin Cancer Res 2014;20:4488-98. [PubMed]
- Spigel DR, Edelman MJ, O’Byrne K, et al. Onartuzumab plus erlotinib versus erlotinib in previously treated stage IIIb or IV NSCLC: Results from the pivotal phase III randomized, multicenter, placebo-controlled METLung (OAM4971g) global trial. J Clin Oncol 2014;32:abstr 8000.
- Aoyama A, Katayama R, Oh-Hara T, et al. Tivantinib (ARQ 197) exhibits antitumor activity by directly interacting with tubulin and overcomes ABC transporter-mediated drug resistance. Mol Cancer Ther 2014;13:2978-90. [PubMed]
- Basilico C, Pennacchietti S, Vigna E, et al. Tivantinib (ARQ197) displays cytotoxic activity that is independent of its ability to bind MET. Clin Cancer Res 2013;19:2381-92. [PubMed]
- Katayama R, Aoyama A, Yamori T, et al. Cytotoxic activity of tivantinib (ARQ 197) is not due solely to c-MET inhibition. Cancer Res 2013;73:3087-96. [PubMed]
- Sequist LV, von Pawel J, Garmey EG, et al. Randomized phase II study of erlotinib plus tivantinib versus erlotinib plus placebo in previously treated non-small-cell lung cancer. J Clin Oncol 2011;29:3307-15. [PubMed]
- Dziadziuszko R. ESMO @ ECC 2013: A Phase III Study of Tivantinib Plus Erlotinib Did Not Meet a Primary Endpoint in Patients with Locally-advanced or Metastatic, Non-squamous NSCLC. ESMO Meeting. 2013.
- Tanizaki J, Okamoto I, Okamoto K, et al. MET tyrosine kinase inhibitor crizotinib (PF-02341066) shows differential antitumor effects in non-small cell lung cancer according to MET alterations. J Thorac Oncol 2011;6:1624-31. [PubMed]
- Ou SH, Kwak EL, Siwak-Tapp C, et al. Activity of crizotinib (PF02341066), a dual mesenchymal-epithelial transition (MET) and anaplastic lymphoma kinase (ALK) inhibitor, in a non-small cell lung cancer patient with de novo MET amplification. J Thorac Oncol 2011;6:942-6. [PubMed]
- Camidge DR, Ou S-HI, Shapiro G, et al. Efficacy and safety of crizotinib in patients with advanced c-MET-amplified non-small cell lung cancer (NSCLC). J Clin Oncol 2014;32:abstr 8001.
- Kurzrock R, Sherman SI, Ball DW, et al. Activity of XL184 (cabozantinib), an oral tyrosine kinase inhibitor, in patients with medullary thyroid cancer. J Clin Oncol 2011;29:2660-6. [PubMed]
- Wakelee HA, Gettinger SN, Engelman JA, et al. A phase Ib/II study of XL184 (BMS 907351) with and without erlotinib (E) in patients (pts) with non-small cell lung cancer (NSCLC). J Clin Oncol 2010;28:abstr 3017.
- Landi L, Minuti G, Salvini J, et al. MET overexpression and gene amplification in NSCLC : a clinical perspective. Dovepress 2013;4:15-25.


