Effects on respiration of nonintubated anesthesia in thoracoscopic surgery under spontaneous ventilation
Authors’ introduction:
Professor Chen (middle right) and Professor Cheng (middle left) lead the nonintubated thoracoscopic surgery program in National Taiwan University Hospital since 2009 with core team members, Dr. Hsu (left) and Dr. Hung (right) (Figure 1).
Introduction
With advancements of one-lung isolation devices and safer one-lung ventilation (OLV) strategies, thoracic surgery evolves progressively in the past decades while intubated general anesthesia with OLV is regarded as a gold standard (1). However, in a modern era of minimally invasive surgery using a video-assisted thoracoscopic surgery (VATS) approach, a renaissance of thoracic surgery without tracheal intubation (i.e., nonintubated) is increasingly re-evaluated in the past decade in efforts to achieve a smoother perioperative course and to offer an opportunity for surgical treatment in patients, for whom an intubated general anesthesia carry high risks (2,3). Encouragingly, nonintubated thoracoscopic procedures have been reported to be feasible and safe for a variety of thoracic diseases, including lung parenchyma resections for lung tumors (4-15) or interstitial lung disease (16), bullaplasty for pneumothorax (17-20), lung volume reduction surgery for emphysema (21-26), decortication for empyema thoracis (27), and mediastinal tumor excisions (28-30).
Nonintubated thoracoscopic procedures are distinct from conventional intubated general anesthesia, by which patients breathe spontaneously under regional anesthesia techniques with or without conscious sedation (31). While tracheal intubation and lung isolation devices are not necessary, neuromuscular blocking drugs and mechanical ventilator are avoided. Aforementioned avoidance of potential adverse effects of intubated general anesthesia may be translated to offer a more physiological, immunological and neuromuscular recovery after surgery, possibly reduce morbidities and therefore shorten length of hospital stay (32-36).
However, creating a surgical pneumothorax in spontaneously breathing patients is not risk free (37). Patients’ oxygenation and ventilation can be impaired because of a loss of lung volume. Therefore, a thorough understanding of pathophysiology of surgical pneumothorax on oxygenation and ventilation is essential to maintain the physiological changes during nonintubated thoracic surgery (36-38). The aim of this review is to look into the factors related to perioperative oxygenation and ventilation during nonintubated thoracic procedures, including surgical pneumothorax, spontaneous breathing, lateral decubitus position, and effects of sedative and analgesic agents on respiratory physiology. Differences in specific patient groups and safety issues related to these physiologic changes are also discussed.
Factors related to oxygenation and ventilation in respiratory physiology
The course of respiration starts with fresh gas enters the lungs with certain rate and volume. The oxygen within fresh gas reaching terminal alveoli diffuses through the alveolar membrane to re-oxygenate the systemic venous blood (i.e., oxygenation). Meanwhile, carbon dioxide contained in systemic venous blood reversely diffuses into alveoli to be exhaled out of body (i.e., ventilation). The fundamental rule of oxygenation and ventilation is the diffusion capacity of an intact alveolar-capillary membrane, where oxygen and carbon dioxide are diffused due to differences of partial pressures of oxygen or carbon dioxide between systemic venous blood and alveoli. To maintain the best of oxygenation, the pulmonary blood flow (Q) and pulmonary ventilation (V) should be matched accordingly. Therefore, any V/Q mismatch can jeopardize pulmonary oxygenation, including low cardiac output, pulmonary thromboembolism, and lung atelectasis or sputum impaction. On the other hand, hypoventilation (a decrease of minute ventilation, which is the product of respiratory rate and tidal volume) can attenuate the elimination of alveolar carbon dioxide, and cause hypercapnia (38,39).
Factors involved in nonintubated VATS
An ideal environment for nonintubated VATS is to create an iatrogenic pneumothorax, a subsequently collapsed lung for adequate surgical exposure. Meanwhile, patients should be maintained in pain-free, stress-free, and effort-less spontaneous ventilation (31,35). To achieve such environment, different regional anesthesia protocols have been applied in nonintubated VATS, including local infiltration (40), intercostal blockades (11), and thoracic epidural anesthesia (34-36). For lengthy procedures such as a lobectomy or a segmentectomy, conscious sedation is usually required due to emotional stress or procedure-related discomfort (6-11).
Factors affecting perioperative oxygenation and ventilation in nonintubated VATS fall into the following categories: surgical pneumothorax, spontaneous breathing, lateral decubitus position, and sedative/analgesic techniques.
Surgical pneumothorax
Surgical pneumothorax starts with the first incision for entry of thoracoscope in the operating hemithorax. By introducing atmospheric pressure into the nondependent pleural cavity, the operated lung collapsed consequently to expose the surgical space (37). Several changes can occur during this process.
Decrease of lung volume
Loss of negative pressure in pleural cavity after surgical pneumothorax causes not only a progressive collapse of the operated lung, but also a decrease of non-operated lung volume because of downward compression of mediastinal tissues by gravity (41). A decrease of total lung volume may lead to hypoventilation.
Decrease of pulmonary perfusion
Surgical pneumothorax can impair the caval blood return because of atmospheric pressure. A decrease of caval venous return leads to low cardiac output and low pulmonary perfusion. However, systemic and pulmonary pressure is usually maintained, probably compensated by an increase of pulmonary vascular resistance from collapsed operated lung and by an intact intrinsic baroreceptor mechanism for a decreasing cardiac output (42). The overall effects of cardiovascular responses to a surgical pneumothorax may therefore be 2-dimensional. An increasing pulmonary vascular resistance of the operated collapsed lung may divert pulmonary blood flow to the dependent lung and improve oxygenation. On the other hand, a decrease of cardiac output may decrease oxygen carrying capacity, resulting low mixed venous oxygen saturation and worsening oxygenation (38,42). It is important to note that use of thoracic epidural anesthesia or sedative drugs may attenuate these intrinsic compensatory mechanisms and should be carefully titrated if adopted (31).
Spontaneous breathing
Preserve of functional residual capacity (FRC) of dependent lung
During conventional intubated general anesthesia, cephalic displacement of diaphragm causes a significant decrease of FRC. Mechanical ventilation with positive end-expiratory pressure is usually required to maintained sufficient ventilation and to avoid of atelectasis of dependent lung. In nonintubated VATS, the patients breathe spontaneously and their diaphragm function and the FRC of dependent lung are less interfered (43,44).
Rebreathing of carbon dioxide and hypercapnia
Without one-lung separation, breathing gases may communicate freely between dependent and nondependent lung during surgical pneumothorax, a phenomenon called “carbon dioxide rebreathing” which leads to hypercapnia (37). While mild to moderate hypercapnia can stimulate the respiratory drive by an increase of breathing rate, use of respiratory depressant agents, such as opioids or sedatives, can aggravate the ventilation and even jeopardize the oxygenation. However, evidences have shown that the degrees of hypoventilation are not clinically significant. The nonintubated VATS procedures are well tolerated by most of patients using a facemask or a nasal cannula to provide supplemental oxygen. In the meanwhile, accumulation of carbon dioxide is usually within the range of “permissive hypercapnia”, which is obviously not harmful perioperatively (2,9,23).
Lateral decubitus position
Mostly, thoracic procedures are performed in a lateral decubitus position, which is usually associated with preferential distribution of breathing gases and pulmonary blood flow in the dependent lung because of pulmonary compliance and gravity. During OLV, these preferential distributions contribute to better matching of ventilation and perfusion, leading to a better oxygenation (41,43).
Effects of intravenous or inhaled anesthetics on oxygenation and ventilation
Nonintubated VATS can be performed in fully awake or moderate-to-deep sedative patients (31,35,37). While awake technique offers neurocognitive monitoring and possibility of cooperation from patients to control their respiratory efforts, various depths of sedation are usually required in anxious patients or lengthy procedures. Both intravenous anesthetics (e.g., propofol, midazolam) and inhaled anesthetics through laryngeal airway masks had been reported with successful results (11-13,34-36). Supplemental oxygen through masks is sufficient to maintain satisfactory oxygen saturation in a relative hypoventilation status. However, it is important to note that both intravenous and inhaled anesthetics induce a decrease in FRC and can be respiratory suppressants (44). Careful titration of sedative depths and monitoring of respiratory rate and exhaled partial pressure of carbon dioxide, such as bi-spectral index monitoring and end-tidal capnography, are important to prevent excessive hypoventilation (Figure 2) (31).
Effects of regional anesthesia techniques on oxygenation and ventilation
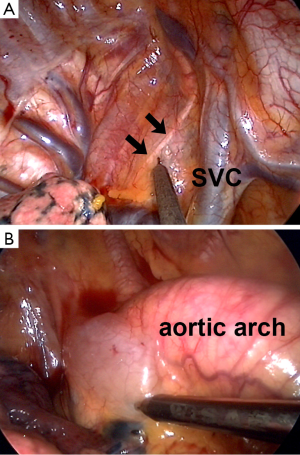
Regional anesthesia had been long reported to be an effective adjuvant to general anesthesia in thoracic surgery. Various approaches, including thoracic epidural anesthesia/analgesia, paravertebral block, intercostal nerve blocks and local wound infiltration, are reported to be effective for analgesia covering chest cage and parietal pleura (45,46). In nonintubated VATS, an effective regional anesthesia to block the unpleasant and painful sensation throughout the surgical wounds are important to prevent patients from emotional irritation, body movement and unfavorable tachypnea after surgical pneumothorax (31). However, the manipulation of lung and traction of hilar structures can cause irritation over visceral pleura, leading to a coughing response and jeopardizing the surgical manipulations (31,36). While visceral afferent transmissions are not blocked by thoracic epidural anesthesia or intercostal blocks, we add intrathoracic vagal blocks to inhibit the visceral irritations and to minimize unwanted cough responses during nonintubated VATS (Figure 3) (6,11,34).
Applying thoracic epidural anesthesia can induce certain degree of motor blockade of respiratory muscles in the thoracic cage, which can lead to around 10% decreases of lung volume, including vital capacity, FRC, forced vital capacity and forced expiratory volume (47,48). Nonetheless, sparing the phrenic nerves, originating for C3-C5 cervical nerve roots, to preserve the diaphragmatic function assures sufficient ventilation despite intercostal muscle paralysis (49). Thoracic epidural anesthesia also induces peripheral vasodilation and functional hypovolemia due to sympathetic blockade (50). On the contrary, intercostal blocks are an effective alternative in place of thoracic epidural anesthesia, which is time-consuming, skill-demanding and requiring more vigilance against potential neurological and cardiorespiratory complications (11).
Oxygenation and ventilation in specific patient groups
Specific patient groups are chosen to elaborate the intraoperative oxygenation and ventilation during nonintubated VATS, including lobectomy for lung cancer patients and a variety of nonintubated VATS procedures for high-risk patients.
Lobectomy for lung cancer patients
In our program, we devote to less invasive surgical and anesthetic management for lung cancer patients (51). Lobectomy through VATS approach is currently the standard treatment for early lung cancer (52). In the past five years, we had performed 238 cases of nonintubated VATS lobectomy, in whom thoracic epidural anesthesia was used in 130 patients and thoracoscope-assisted intercostal blocks in 108 patients. We preferred to sedate our patients to induce a relative hypoventilation using incremental opioid and to attenuate cough reflex effectively through intrathoracic vagal blocks (11,31). Our patients underwent nonintubated VATS were mostly female (71%) with short-stature (mean body weight index: 22.9 kg/m2) and normal lung function (forced expiratory volume in 1 second: 111.7% of predicted). During OLV after surgical pneumothorax, the mean partial pressure of oxygen (PaO2) was 140.4 mmHg while the mean partial pressure of carbon dioxide was 47.7 mmHg. Although there were 20 patients (8.8%) experienced oxygen desaturation below 90% sometime, simple oxygen supplement through facemasks were sufficient to correct their transient arterial desaturation, except in one patients requiring conversion to tracheal intubation because of persistent low oxygen saturation below 80%.
Nonintubated VATS procedures in high-risk patients
One of the major advantages of nonintubated VATS is to offer surgical opportunities for patients who are too risky for an intubated general anesthesia (19,36,53). Although appealing, sufficiency of respiratory status during surgical pneumothorax is highly concerned, especially in patients with compromised pulmonary functions. To address these issues, Drs. Mineo and Pompeo from Awake Thoracic Surgery Study Group had performed a series of compromised patients for different nonintubated VATS procedures, including pleurodesis for malignant pleural effusion (54), decortication for empyema thoracis (27), thoracoscopic biopsy for pulmonary interstitial disease (16) and lung volume reduction surgery for severe emphysema (23-26). Reported results were also satisfactory that oxygenation could be easily maintained during surgical pneumothorax when patients were kept awake. Asymptomatic hypercapnia were usually noted in patients with severe emphysema but resolved after procedures (23).
Safety issues regarding nonintubated VATS
Coughing
Coughing is a protective reflex against entry of foreign bodies into the tracheobronchial airway. It is usually abolished in an intubated general anesthesia because of neuromuscular blockade. However, it can be triggered from excessive stretch of lung parenchyma or hilar manipulation of bronchial structure during a nonintubated VATS (36). Unexpected coughing during nonintubated thoracic procedures not only impedes the surgical exposure, but also potentially causes life-threatening injuries to intra-thoracic structures (6). In unexpected or intractable cough cases, a conversion to intubated general anesthesia may be required. Therefore, it is mostly advised to manipulate the lung parenchyma and hilar structures gently to avoid of cough reflex during nonintubated VATS, but it is usually inevitable in major and lengthy procedures such as nonintubated lobectomy or segmentectomy (10,26). Methods have been proposed using aerosol lidocaine inhalation (53), stellate ganglion block (55) and intrathoracic vagal nerve block (11,34) to attenuate coughing reflex during nonintubated VATS.
In our experiences, we prefer vagal nerve block to inhibit cough reflex, where infiltration of local anesthetics can be produced at the level of the lower tracheal for right-sided operations and at the level of aortopulmonary window for left-sided operations (Figure 3) (11,31,51). The duration of vagal blockade can last more than 3 hours. Repeat dose can be given in prolonged operations. In most cases, the effect of vagal block has worn off after non-intubated VATS. Occasionally, patients undergoing short operations may experience hoarseness in the recovery area, which may suggest residual vagal blockade. It is noteworthy that patients with residual vagal blockade are not recovered sufficiently to protect themselves from aspiration. Oral consumption should therefore be delayed until cough reflex is fully recovered (31).
Conversion to conventional intubated general anesthesia
A thorough plan B for conversion to an intubated general anesthesia should always be prepared in advance for the safety practice of nonintubated VATS (2). Despite of careful patient selection and vigilant monitoring, intraoperative conversion to tracheal intubation is occasionally inevitable, mostly resulting from significant mediastinal movement, persistent hypoxemia, unexpected dense pleural adhesions, and significant bleeding due to vascular injury, or insufficient anesthesia/analgesia (6-11,51). Intraoperative conversion to tracheal intubation in a lateral decubitus position can be a technical challenge to anesthesiologists. While direct laryngoscopy for intubation is feasible, a video-assisted laryngoscope, fiberoptic bronchoscope, or intubating laryngeal mask airway may be more convenient instruments for tracheal intubation in lateral decubitus position. Lung separation can then be achieved using an endobronchial blocker to occlude the operated lung. Otherwise, the patients can also be replaced in a supine position for tracheal intubation in usual manners at the discretion of the caring anesthesiologist (31).
In our practices, when conversion to tracheal intubation is indicated as aforementioned situations occur, the surgical wounds would first be sealed with transparent waterproof dressings after insertion of a chest tube to re-expand the operated lung. Meanwhile, a single lumen tracheal tube will be inserted under the guidance of fiberoptic bronchoscope, followed by insertion of a bronchial blocker without changing the patient’s position. Collaboration between surgeon and anesthesiologist is essential for nonintubated VATS, especially in situations of tracheal conversion (11,51).
Conclusions
It is still early to conclude the roles of nonintubated techniques in thoracic surgery. However, encouraging and initial results have broadened our knowledge and experiences in various nonintubated VATS procedures and in a wide variety of patients groups. Hypoventilation during surgical pneumothorax is inevitable, but oxygenation and ventilation during nonintubated VATS are manageable and clinically tolerable. Even so, an understanding of respiratory changes during surgical pneumothorax and a well-prepared conversion protocol are essential to guarantee perioperative safety, especially in sedative patients.
Acknowledgements
Funding: This work is supported by research grants from National Taiwan University Hospital (NTUH104-P08 to Dr. Chen JS) and Taiwan Lung Foundation, Taipei, Taiwan.
Disclosure: The authors declare no conflict of interest.
References
- Brodsky JB, Cohen E. Video-assisted thoracoscopic surgery. Curr Opin Anaesthesiol 2000;13:41-5. [PubMed]
- Pompeo E. State of the art and perspectives in non-intubated thoracic surgery. Ann Transl Med 2014;2:106. [PubMed]
- Hung MH, Hsu HH, Cheng YJ, et al. Nonintubated thoracoscopic surgery: state of the art and future directions. J Thorac Dis 2014;6:2-9. [PubMed]
- Pompeo E, Mineo D, Rogliani P, et al. Feasibility and results of awake thoracoscopic resection of solitary pulmonary nodules. Ann Thorac Surg 2004;78:1761-8. [PubMed]
- Pompeo E, Mineo TC. Awake pulmonary metastasectomy. J Thorac Cardiovasc Surg 2007;133:960-6. [PubMed]
- Chen JS, Cheng YJ, Hung MH, et al. Nonintubated thoracoscopic lobectomy for lung cancer. Ann Surg 2011;254:1038-43. [PubMed]
- Tseng YD, Cheng YJ, Hung MH, et al. Nonintubated needlescopic video-assisted thoracic surgery for management of peripheral lung nodules. Ann Thorac Surg 2012;93:1049-54. [PubMed]
- Wu CY, Chen JS, Lin YS, et al. Feasibility and safety of nonintubated thoracoscopic lobectomy for geriatric lung cancer patients. Ann Thorac Surg 2013;95:405-11. [PubMed]
- Chen KC, Cheng YJ, Hung MH, et al. Nonintubated thoracoscopic lung resection: a 3-year experience with 285 cases in a single institution. J Thorac Dis 2012;4:347-51. [PubMed]
- Hung MH, Hsu HH, Chen KC, et al. Nonintubated thoracoscopic anatomical segmentectomy for lung tumors. Ann Thorac Surg 2013;96:1209-15. [PubMed]
- Hung MH, Hsu HH, Chan KC, et al. Non-intubated thoracoscopic surgery using internal intercostal nerve block, vagal block and targeted sedation. Eur J Cardiothorac Surg 2014;46:620-5. [PubMed]
- Guo Z, Shao W, Yin W, et al. Analysis of feasibility and safety of complete video-assisted thoracoscopic resection of anatomic pulmonary segments under non-intubated anesthesia. J Thorac Dis 2014;6:37-44. [PubMed]
- Ambrogi MC, Fanucchi O, Korasidis S, et al. Nonintubated thoracoscopic pulmonary nodule resection under spontaneous breathing anesthesia with laryngeal mask. Innovations (Phila) 2014;9:276-80. [PubMed]
- Hung MH, Cheng YJ, Chan KC, et al. Nonintubated uniportal thoracoscopic surgery for peripheral lung nodules. Ann Thorac Surg 2014;98:1998-2003. [PubMed]
- Hung MH, Cheng YJ, Hsu HH, et al. Nonintubated uniportal thoracoscopic segmentectomy for lung cancer. J Thorac Cardiovasc Surg 2014;148:e234-5. [PubMed]
- Pompeo E, Rogliani P, Cristino B, et al. Awake thoracoscopic biopsy of interstitial lung disease. Ann Thorac Surg 2013;95:445-52. [PubMed]
- Pompeo E, Tacconi F, Mineo D, et al. The role of awake video-assisted thoracoscopic surgery in spontaneous pneumothorax. J Thorac Cardiovasc Surg 2007;133:786-90. [PubMed]
- Noda M, Okada Y, Maeda S, et al. Successful thoracoscopic surgery for intractable pneumothorax after pneumonectomy under local and epidural anesthesia. J Thorac Cardiovasc Surg 2011;141:1545-7. [PubMed]
- Mukaida T, Andou A, Date H, et al. Thoracoscopic operation for secondary pneumothorax under local and epidural anesthesia in high-risk patients. Ann Thorac Surg 1998;65:924-6. [PubMed]
- Pompeo E, Tacconi F, Frasca L, et al. Awake thoracoscopic bullaplasty. Eur J Cardiothorac Surg 2011;39:1012-7. [PubMed]
- Pompeo E, Mineo TC. Two-year improvement in multidimensional body mass index, airflow obstruction, dyspnea, and exercise capacity index after nonresectional lung volume reduction surgery in awake patients. Ann Thorac Surg 2007;84:1862-9; discussion 1862-9.
- Tacconi F, Pompeo E, Forcella D, et al. Lung volume reduction reoperations. Ann Thorac Surg 2008;85:1171-7. [PubMed]
- Pompeo E, Rogliani P, Tacconi F, et al. Randomized comparison of awake nonresectional versus nonawake resectional lung volume reduction surgery. J Thorac Cardiovasc Surg 2012;143:47-54, 54.e1.
- Pompeo E, Tacconi F, Mineo TC. Comparative results of non-resectional lung volume reduction performed by awake or non-awake anesthesia. Eur J Cardiothorac Surg 2011;39:e51-8. [PubMed]
- Tacconi F, Pompeo E, Mineo TC. Duration of air leak is reduced after awake nonresectional lung volume reduction surgery. Eur J Cardiothorac Surg 2009;35:822-8; discussion 828. [PubMed]
- Mineo TC, Pompeo E, Mineo D, et al. Awake nonresectional lung volume reduction surgery. Ann Surg 2006;243:131-6. [PubMed]
- Tacconi F, Pompeo E, Fabbi E, et al. Awake video-assisted pleural decortication for empyema thoracis. Eur J Cardiothorac Surg 2010;37:594-601. [PubMed]
- Tsunezuka Y, Oda M, Matsumoto I, et al. Extended thymectomy in patients with myasthenia gravis with high thoracic epidural anesthesia alone. World J Surg 2004;28:962-5: discussion 965-6.
- Matsumoto I, Oda M, Watanabe G. Awake endoscopic thymectomy via an infrasternal approach using sternal lifting. Thorac Cardiovasc Surg 2008;56:311-3. [PubMed]
- Pompeo E, Tacconi F, Mineo TC. Awake video-assisted thoracoscopic biopsy in complex anterior mediastinal masses. Thorac Surg Clin 2010;20:225-33. [PubMed]
- Yang JT, Hung MH, Chen JS, et al. Anesthetic consideration for nonintubated VATS. J Thorac Dis 2014;6:10-3. [PubMed]
- Vanni G, Tacconi F, Sellitri F, et al. Impact of awake videothoracoscopic surgery on postoperative lymphocyte responses. Ann Thorac Surg 2010;90:973-8. [PubMed]
- Tacconi F, Pompeo E, Sellitri F, et al. Surgical stress hormones response is reduced after awake videothoracoscopy. Interact Cardiovasc Thorac Surg 2010;10:666-71. [PubMed]
- Liu J, Cui F, Li S, et al. Nonintubated video-assisted thoracoscopic surgery under epidural anesthesia compared with conventional anesthetic option: a randomized control study. Surg Innov 2015;22:123-30. [PubMed]
- Kao MC, Lan CH, Huang CJ. Anesthesia for awake video-assisted thoracic surgery. Acta Anaesthesiol Taiwan 2012;50:126-30. [PubMed]
- Mineo TC. Epidural anesthesia in awake thoracic surgery. Eur J Cardiothorac Surg 2007;32:13-9. [PubMed]
- Pompeo E. Awake thoracic surgery--is it worth the trouble? Semin Thorac Cardiovasc Surg 2012;24:106-14. [PubMed]
- Levin AI, Coetzee JF, Coetzee A. Arterial oxygenation and one-lung anesthesia. Curr Opin Anaesthesiol 2008;21:28-36. [PubMed]
- Kelman GR, Nunn JF, Prys-Roberts C, et al. The influence of cardiac output on arterial oxygenation: a theoretical study. Br J Anaesth 1967;39:450-8. [PubMed]
- Katlic MR, Facktor MA. Video-assisted thoracic surgery utilizing local anesthesia and sedation: 384 consecutive cases. Ann Thorac Surg 2010;90:240-5. [PubMed]
- Grichnik KP, Clark JA. Pathophysiology and management of one-lung ventilation. Thorac Surg Clin 2005;15:85-103. [PubMed]
- Li TH, Rheinlander HF, Etsten B. Circulatory changes due to open pneumothorax in surgical patients. Anesthesiology 1960;21:171-7. [PubMed]
- Lohser J, Ishikawa S. Physiology of thelateral decubitus Position, open chest and one-lung Ventilation. In: Slinger P. eds. Principles and practice of anesthesia for thoracic surgery. New York: Springer Science, 2011:71-82.
- Hedenstierna G, Edmark L. The effects of anesthesia and muscle paralysis on the respiratory system. Intensive Care Med 2005;31:1327-35. [PubMed]
- Fortier S, Hanna HA, Bernard A, et al. Comparison between systemic analgesia, continuous wound catheter analgesia and continuous thoracic paravertebral block: a randomised, controlled trial of postthoracotomy pain management. Eur J Anaesthesiol 2012;29:524-30. [PubMed]
- Manion SC, Brennan TJ. Thoracic epidural analgesia and acute pain management. Anesthesiology 2011;115:181-8. [PubMed]
- Takasaki M, Takahashi T. Respiratory function during cervical and thoracic extradural analgesia in patients with normal lungs. Br J Anaesth 1980;52:1271-6. [PubMed]
- Groeben H. Epidural anesthesia and pulmonary function. J Anesth 2006;20:290-9. [PubMed]
- Warner DO, Warner MA, Ritman EL. Human chest wall function during epidural anesthesia. Anesthesiology 1996;85:761-73. [PubMed]
- Clemente A, Carli F. The physiological effects of thoracic epidural anesthesia and analgesia on the cardiovascular, respiratory and gastrointestinal systems. Minerva Anestesiol 2008;74:549-63. [PubMed]
- Chen KC, Cheng YJ, Hung MH, et al. Nonintubated thoracoscopic surgery using regional anesthesia and vagal block and targeted sedation. J Thorac Dis 2014;6:31-6. [PubMed]
- Rueth NM, Andrade RS. Is VATS lobectomy better: perioperatively, biologically and oncologically? Ann Thorac Surg 2010;89:S2107-11. [PubMed]
- Guarracino F, Gemignani R, Pratesi G, et al. Awake palliative thoracic surgery in a high-risk patient: one-lung, non-invasive ventilation combined with epidural blockade. Anaesthesia 2008;63:761-3. [PubMed]
- Mineo TC, Sellitri F, Tacconi F, et al. Quality of life and outcomes after nonintubated versus intubated video-thoracoscopic pleurodesis for malignant pleural effusion: comparison by a case-matched study. J Palliat Med 2014;17:761-8. [PubMed]
- Al-Abdullatief M, Wahood A, Al-Shirawi N, et al. Awake anaesthesia for major thoracic surgical procedures: an observational study. Eur J Cardiothorac Surg 2007;32:346-50. [PubMed]



