Dietary, non-microbial intervention to prevent Helicobacter pylori-associated gastric diseases
Introduction
Helicobacter pylori (H. pylori) infection has commonly been accepted to be a cause of acute and chronic gastritis, gastric and duodenal ulcer, gastric mucosa-associated lymphoid tissue (MALT) lymphoma and gastric adenocarcinoma as well as to be associated with extragastric diseases such as iron deficiency anemia, idiopathic thrombocytopenia, atherosclerosis and chronic headache, etc. (1). Particularly, supported with the fact that recurrence or complication of peptic ulcer and several extra-gastric associated diseases can be treated by eradication therapy as well as the result that H. pylori could be a direct cause of epigastric pain in functional gastric disorder, H. pylori infection has been thought to be an important infectious bacteria as a big etiology of various gastric diseases as well as bacteria satisfying evidence based medicine (2).
In spite of these positive associations, the biggest part among the several unmet medical needs which have been controversial until now is “the association with development of gastric cancer”. In various molecular and biologic studies (3) using in vitro and in vivo models, H. pylori infection has been reported as a direct or promoting factor for gastric cancer development. Also previous clinical cohort or clinical studies proved that gastric cancer development was significantly associated with H. pylori infection (4) as well as that gastric cancer recurrence rate was significantly lower in H. pylori eradication group after gastric mucosal resection (5). In addition, based on the diverse strong evidences that H. pylori eradication can be a method of gastric cancer prevention, starting from 2013 in Japan, national wide strategy that all national people with chronic gastritis infected with H. pylori should get the eradication therapy has been under progress (6). However, the approach to make guideline of gastric disease treatment with H. pylori eradication has been concluded to be very careful because of several reasons such as the opinion that H. pylori eradication is not necessary considering the over 50% of incidence in world’s population, the report that only partial people group show efficacy of H. pylori eradication, the appearance of antibiotic resistant bacteria strain and the opposite opinion (7) of some researchers who accept H. pylori as a commensal bacteria.
Recently, based on the results that inflammation is important pathogenesis between H. pylori infection and gastric cancer development and that some of chronic atrophic gastritis is reversible and regeneration to non-atrophic condition is possible, increasing opinion arise that several plant nutrients, probiotics and anti-oxidants which have no side effect in long term administration can suppress the gastric carcinogenesis as well as impose significant regenerative effects (8). Based on hypotheses (8) that diverse food nutrients can reduce inflammation induced by H. pylori infection, eradication can reduce atrophic gastritis based on patient’s self-regeneration ability and the accumulation of these phenomena can ultimately prevent gastric cancer development, several preclinical and clinical trial studies regarding disease prevention through dietary nutrients are currently in progress. Those studies will be introduced in this review article (Figure 1). As examples, Korean red ginseng and special form of licorice (clinical trials have already been finished for both of them) as well as development of cancer-preventive Korea Kimchi based on material which was proven to have suppressive effect on gastric cancer development will be introduced in this review.
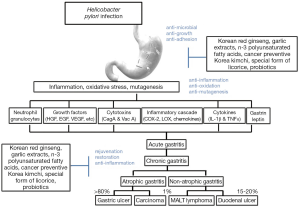
The effect of Korean red ginseng on H. pylori infection: augmented eradication rate and rejuvenating action of atrophic gastritis
H. pylori infection was the main risk factor of chronic gastritis and gastroduodenal ulcers (1,2), International Agency for Research on Cancer (IARO) defined the H. pylori infection was group I carcinogen in 1994 and revised version was released at 2014 (3). H. pylori infection induced chronic inflammation, and provided tumor microenvironment crucial to cancer initiation, survival of tumor cell, progression of cancer, so chronic inflammation had a key role on H. pylori-associated gastric cancer. Therefore, it was important to eradicate H. pylori infection or attenuate chronic inflammation for prevention of H. pylori-associated gastric cancer. The Korean red ginseng attenuated 5-lipoxygenase (LOX) activity, which is important mechanism on carcinogenesis. In other words, long term application of the Korean red ginseng expected suppression of development of H. pylori-associated gastric cancer (9). Also, extracts of Korean red ginseng had preventive effect of gastric mucosa cytotoxicity, which interrupted apoptosis by blocking receptor signaling pathway on H. pylori induced gastric mucosal injury, reduced binding of NF-κB with DNA. Until the present, it was known that the efficacy of Korean red ginseng was anti-oxidative, anti-inflammatory effect (10-13), these effects were based on the facts that ginsenoside Rb1 suppressed the expression of tumor necrosis factor alpha (TNF-α) induced by lipopolysaccharide (14), and the acidic polysaccharide, panaxytriol interrupted H. pylori colonization and attachment on gastric mucosa (12,15). Extracts of Korean red ginseng suppressed production of hydrogen sulfide (H2S) on H. pylori infected gastric mucosa, then prevented significant mucosal injury, inhibited halitosis, suppressed carcinogenesis-related angiogenesis (16,17). Through clinical trial, the authors investigated the efficacy of Korean red ginseng on H. pylori eradication rate, and confirmed significant improvement of eradication rate after 10 weeks treatment of Korean red ginseng after conventional H. pylori eradication regimen. In conclusion, the application of Korean red ginseng improved the eradication rate and was superior in quality, in order to receive recognition as a guideline, additional large scale randomized prospective trials were needed.
The effect of garlic extracts on H. pylori infection: anti-inflammatory and anti-mutagenic activity
Garlic (Allium sativum L.) is one of the most widely grown vegetable crops in Asia including Korea. Garlic has long been used as a seasoning or condiment for its pungent flavor for a long time. Garlic is also known as a medicinal food ingredient with physiological potential since ancient times (18). Garlic contains at least 33 sulfur compounds such as allicin, alliin, ajoene, diallyl trisulfide (DATS) and others. The sulfur compounds are responsible many of its medicinal effects; antioxidant, antiinflammation, antimutagenic, haematological, antimicrobial, hepatoprotective and antineoplastic properties. In particular, oil-soluble sulfur compounds [e.g., sulfoxides (alliin), diallyl sulfide (DAS), diallyl disulfide (DADS), DATS] and water-soluble sulfur compounds, e.g., S-allylcysteine (SAC) and S-allyl mercaptocysteine, have all been shown to exhibit antimicrobial, antioxidant and antiinflammation activity (19). In recent, several studies reported that sulfur compounds have effects to inhibit H. pylori colonization, decrease gastric inflammation and oxidative stress by H. pylori infection, and lead gastric ulcer healing. Many preparations of garlic with different compositions of bioactive compounds are commercially available (20,21). Among these preparations (water, acetone, ethanol, and hexane), ethanol and acetone extracts showed the highest bacteriostatic activities against H. pylori (22). Garlic oil extracts also exhibited direct anti-H. pylori effects (23). Antimicrobial activity of the DAS increased with the number of sulfur atoms (24). Allicin and methyl-allyl thiosulfinate from acetonic garlic extracts have shown inhibition of the in vitro growth of H. pylori (25). In vivo study, garlic extract prevents H. pylori-induced gastritis in Mongolian gerbil model (26). In clinical reports, Gail et al. (27,28) showed that the ingestion of garlic supplement significantly reduce the prevalence of gastritis in Linqu County, Shandong Province, China, a region with high gastric cancer mortality rates and a prevalence in adults of H. pylori of approximately 67%. Fani et al. (29) suggested combined garlic and omeprazole instead of the standard quadruple therapy for the eradication of H. pylori infection (29). In our recent study, SAC, a water-soluble garlic compounds that had been known to possess a powerful anti-oxidant property, has been shown to exert antiinflammatory and mucosa protective effects against gastrointestinal (GI) inflammation. Especially, SAC significantly prevents H. pylori-induced gastritis in vivo. SAC significantly inhibited pro-inflammatory signaling, including cyclooxygenase-2 (COX-2), TNF-α, interleukin-1 beta (IL-1β) and interleukin-6 (IL-6), and decreased in macrophage infiltration as well as gastric cell proliferation. Moreover, SAC suppressed in vitro growth of H. pylori. Finally, these results suggest that garlic extracts and compounds can be a gastroprotective agent against H. pylori-induced gastric mucosal damage and gastric cancer.
The preventive effects of omega-3 polyunsaturated fatty acids (n-3 PUFAs) on H. pylori infection-associated atrophic gastritis and gastric tumorigenesis
Omega-3 is polyunsaturated fatty acids (n-3 PUFAs) with a double bond at the third carbon atom from the end of the carbon chain. It is known to prevent anti-cancer, anti-oxidant and anti-inflammation (30). The three types of n-3 PUFAs involved in human physiology are α-linolenic acid (ALA), docosahexaenoic acid (DHA) and eicosapentaenoic acid (EPA). Common sources of plant oils containing the n-3 ALA FA include walnut and seed oil, while sources of animal n-3 EPA and DHA FAs include fish oils and egg oils. Since mammals can’t synthesize n-3, we must get n-3 from drugs or foods (31). Many research groups studied about between n-3 and cardiovascular diseases who reported that n-3 PUFAs have effects for function recovery of the coronary arteries, plaque reduction of atherosclerosis and inhibition of inflammatory cytokines which take effects through inhibition of NF-κB signaling (30-32). Recently several investigators have examined the anti-inflammatory effects of n-3 on H. pylori-induced gastric diseases because n-3 FAs affect lipid raft assembly and its functions by reducing membrane fluidity of bacteria (32,33). Lipid raft is microdomain having abundant cholesterol of cell membrane which is essential for anti-inflammatory action because NF-κB activation dependent inflammation actively occurred after H. pylori infection (34). Correia et al. (35) reported that DHA significantly decreased the inflammation of gastric mucosa by reducing growth of H. pylori in dose-dependently in vivo and in vitro. All of these data suggested that DHA is possible as supplementary drug on either removal treatment of H. pylori or attenuation of inflammation. However, Meier et al. (36) showed that removal treatment of H. pylori with n-3 have no statistically significant compare with general removal treatment methods. Therefore our study group investigated effects of n-3 FAs in H. pylori induced gastritis. n-3 PUFAs decreased oxidative stress and inflammation induced by H. pylori infection in normal gastric mucosa cells. To check the effects for anti-inflammation and anti-cancer of n-3 PUFAs in H. pylori infection we performed long period animal model. In 45 weeks after H. pylori infection, wild type mice indicated not only gastric cancer formation with atrophic gastritis but also autophagy and lipid raft disturbance more than n-3 PUFAs treated mice. Recently, Kuriki et al. (37) suggested that there is no correlation between erythrocyte composition and disease incidence of gastric cancer in human study. Conclusively, in order to demonstrate the preventive or therapeutic effects of n-3 PUFAs for gastric diseases induced by H. pylori infection, large scale clinical and detailed mechanism study. If we have results about that long-term administration of n-3 PUFAs have preventive effects for gastritis and H. pylori-induced tumorigenesis, might be an example of cancer prevention through foods.
Cancer preventive—kimchi
Korea and Japan are similar to gastric carcinogenesis environment. In Japan, the eradication of H. pylori from patients with chronic gastritis as a way of cancer prevention start the effort to reduce the occurrence of cancer from this year. Kimchi is representative fermented, antioxidant food and a safe food for thousands of years been ingested in Korea. If we can make a specially reciped kimchi, it hypothesized this would be an ideal food with cancer prevention. The following experiment was in our laboratory. Several researchers have found that Lactobacillus plantarum (L. plantarum) derived from kimchi can promote suppressor of cytokine signaling (SOCS) secretion (38,39). To explore the effects of specially reciped kimchi containing phytoceutical on H. pylori, H. pylori infected wild type mice were sacrificed after 24 weeks and after 36 weeks. Among them, one group was fed drinking water containing extract of cancer preventive kimchi. As a result, H. pylori infection group was found severe inflammation, mucosal erosion, dysplasia, adenoma of the stomach. However, the group fed with cancer preventive kimchi was reduced significantly. Furthermore, H. pylori infection group increased COX-2 expression, NF-κB and p-STAT3. But redesigned kimchi group reduced COX-2, NF-κB and p-STAT3 expression and increased antioxidant enzyme such as heme oxygenase-1 (HO-1) compared to H. pylori infection group. In addition, we confirmed that a specially reciped kimchi reduced H. pylori-induced inflammation enzyme; COX-2, inducible nitric oxide synthase (iNOS), TNF-α, and induced apoptosis to gastric cancer cell specifically. Taken with all these evidences, it is expected to possible cancer preventive kimchi development for gastric cancer prevention specially formulated with well-known anti-cancer phytochemicals.
Special form of licorice for H. pylori infection
Licorice extracts derived from dried root of Glycyrrhiza species, which is widely used as drugs in East and West are known to be have preventive effects for GI disease. Licorice extracts contained biologically active substance including glycyrrhizin, licochalcone A, licorisoflavan A etc. (40). Recently several research groups suggested that licorice extracts and its biologically active substance have effects of detoxification, antioxidant, anti-inflammation, anti-bacteria and anti-cancer. Nevertheless licorice whole extracts were limited to use due to the side effects by glycyrrhizin. Therefore our research group studied that special licorice extracts (s-lico) have preventive effects for H. pylori induced gastritis in vitro and in vivo. S-lico contained low glycyrrhizin and high lico. When stomach was infected by H. pylori, generally ROS production was increased which affect inflammation and tumorigenesis in stomach. Thus, we measured ESR (electron spin resonance) and dichlorofluorescin diacetate (DCFDA) to check the ROS-scavenging activity of s-lico in vitro. As a result, s-lico had significant scavenging activity for ROS and H. pylori treated cells showed that ROS generation was increased but H. pylori and s-lico (10 µg/mL) treated cells were decreased ROS generation in DCFDA. Therefore s-lico had ROS-scavenging activity through anti-oxidant effects against H. pylori infection. Moreover we examined inflammatory associated factors including COX-2, iNOS and TNF-α. After H. pylori infection, inflammatory factors were increased whereas s-lico decreased the expression of COX-2, iNOS and TNF-α in vitro. Several studies reported that H. pylori infection induced inflammation and tumor by strong angiogenesis activity in stomach. So, to determine that s-lico had effects for angiogenesis, we checked the VEGF expression as known angiogenesis marker in cancer cells. As a result s-lico exposed cells significantly declined the expression of VEGF. It is important that s-lico decreased angiogenesis activity by H. pylori infection so s-lico was useful as potential candidate material in augmented angiogenesis activity of cancer cells. To demonstrate whether preventive effects of s-lico validated in vivo, we performed animal experiment using mice. Wild type mice were infected by H. pylori and then sacrificed after 24 weeks. We divided three groups including non-treated group, H. pylori infected group and diet contained s-lico intake group with H. pylori infection. As a result, H. pylori infected group showed higher score of inflammation, mucosal erosion, dysplasia and adenocarcinoma in stomach but s-lico intake group indicated remarkably lower level about stomach lesion. And H. pylori group elevated the expressions of basic fibroblast growth factor (bFGF), intercellular adhesion molecule 1 (ICAM1), TNF-related activation induced cytokine (TRANCE) and TNF receptor superfamily, member 19 (TNFRSF19/TROY) as angiogenesis associated cytokines. On the other hand, s-lico treated group reduced the levels of angiogenesis related cytokines. The COX-2 expression was increased in H. pylori group and prostaglandin E2 (PGE2) as product of COX-2 also was high expression. However, s-lico intake group decreased COX-2 and PGE2 levels together with inflammatory cytokines including TNF-α, IL-1β and IL-6 etc. Taken together, we supported that s-lico had effects of anti-oxidant, anti-inflammation and anti-cancer in inflammation related carcinogenesis with gastritis thus, s-lico may affects preventive effects for stomach disease by H. pylori infection.
Probiotics as alternative therapies for H. pylori infection
Probiotics, non-pathogenic microbial feed, are already being widely applied in the treatment of GI infections and GI diseases including irritable bowel syndrome, inflammatory bowel disease, pancreatitis, and liver diseases as an alternate way to enhance anti-microbial and anti-inflammation (41-45). In clinical reports, the addition of probiotics to proton pump inhibitor (PPI)-based triple therapy augmented the likelihood of successful H. pylori eradication. In particular, probiotics competed directly with H. pylori as well as reduced H. pylori associated inflammation (46-48). L. salivarius significantly decreased gastric epithelial cell chemokine, such as interleukin-8 (IL-8), production responses to H. pylori infection. L. acidophilus reduced H. pylori-induced inflammation through the inactivation of the Smad7 and NF-κB pathways. L. plantarum, L. rhamnosis and L. acidophilus all could ameliorate H. pylori-induced inflammation by either efficiently activating SOCS expression or inactivating the janus kinase 2 (JAK2) signaling pathways. Several human studies have investigated the efficacy of probiotics for the treatment of H. pylori infection (49-51). Most of the studies reported an improvement of H. pylori gastritis and decrease in H. pylori colonization after administration of probiotics. However, none of the studies could demonstrate complete eradication of H. pylori by probiotic alone (45,52,53). In a recent, a growing body of experimental and clinical evidence has supported the importance of intestinal microbiota, in H. pylori infection as well as the onset of GI diseases. Probiotics, as live organisms, can modulate the human microbiota and promote health, prevent antibiotic side effects, stimulate the immune response and directly compete with pathogenic bacteria (54). L. species are acid-resistant and commensal and their concentrations in the normal human stomach vary between 0 and 103 mL−1. They can survive in the stomach for periods of up to 2 h. Therefore, the administration of probiotics, such as Lactobacillus species, can perturb microbiota in the H. pylori infected stomach. Finally, the advantages attributed to probiotics in H. pylori infection, such as improvement of the eradication rate, reduction of side effects associated with eradication drugs, and some direct anti-inflammatory action, may represent only a small part of their advantages. Extensive investigation of the microbiota relevant to H. pylori infection will be required to elucidate additional mechanisms and relationships.
Conclusions
Modern evidence-based medicine (EBM) has big difference from the traditional oriental medicine only based on experiences. In other words, noble medical technology can be available through strict verification of clinical trial with double blind condition (55). The verification of multi-centers through long term observation is necessary for food nutritional approach for the prevention of H. pylori associated diseases through the control of H. pylori infection. In addition to guideline for H. pylori eradication, long term approach with food nutrients could be a noble treatment method to solve the unmet medical needs related with H. pylori infection (Figure 1). Particularly, cancer-preventive Korea kimchi which we are recently developing as well as all national people eradication plan in Japan from 2013 deserve to have a trial considering that the incidence of gastric cancer is continuous and mortality rate of that is about 20 per 100,000 population (in Korea) and about 50 per 100,000 population (in Japan), which clearly means novel approach could reduce the mortality rate of gastric cancer in these high risk countries. Korean red ginseng has been proven to have safety because Korean people have had it over 100 years, which is similar to a kind of clinical trial. Therefore, the clear evidence based medical (EBM) approach grounded on well constituted clinical trial plan is needed on plausibility of H. pylori control by these food nutrients.
Acknowledgements
Funding: This research was supported by the National Center of Efficacy Evaluation for the Development of Health Products Targeting Digestive Disorders (NCEED) grant (A102063) from the Ministry of Health and Welfare and by Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education (2014R1A1A2058732).
Disclosure: The authors declare no conflict of interest.
References
- Marshall BJ, Warren JR. Unidentified curved bacilli in the stomach of patients with gastritis and peptic ulceration. Lancet 1984;1:1311-5. [PubMed]
- Hentschel E, Brandstatter G, Dragosics B, et al. Effect of ranitidine and amoxicillin plus metronidazole on the eradication of Helicobacter pylori and the recurrence of duodenal ulcer. N Engl J Med 1993;328:308-12. [PubMed]
- Schistosomes, liver flukes and Helicobacter pylori. IARC Working Group on the Evaluation of Carcinogenic Risks to Humans. Lyon, 7-14 June 1994. IARC Monogr Eval Carcinog Risks Hum 1994;61:1-241. [PubMed]
- Uemura N, Okamoto S, Yamamoto S, et al. Helicobacter pylori infection and the development of gastric cancer. N Engl J Med 2001;345:784-9. [PubMed]
- Fukase K, Kato M, Kikuchi S, et al. Effect of eradication of Helicobacter pylori on incidence of metachronous gastric carcinoma after endoscopic resection of early gastric cancer: an open-label, randomised controlled trial. Lancet 2008;372:392-7. [PubMed]
- Lee SY. Current progress toward eradicating Helicobacter pylori in East Asian countries: differences in the 2013 revised guidelines between China, Japan, and South Korea. World J Gastroenterol 2014;20:1493-502. [PubMed]
- Sachs G, Scott DR. Helicobacter pylori: Eradication or Preservation. F1000 Med Rep 2012;4:7. [PubMed]
- Shiotani A, Cen P, Graham DY. Eradication of gastric cancer is now both possible and practical. Semin Cancer Biol 2013;23:492-501. [PubMed]
- Park S, Yeo M, Jin JH, et al. Inhibitory activities and attenuated expressions of 5-LOX with red ginseng in Helicobacter pylori-infected gastric epithelial cells. Dig Dis Sci 2007;52:973-82. [PubMed]
- Liu CX, Xiao PG. Recent advances on ginseng research in China. J Ethnopharmacol 1992;36:27-38. [PubMed]
- Shao ZH, Xie JT, Vanden Hoek TL, et al. Antioxidant effects of American ginseng berry extract in cardiomyocytes exposed to acute oxidant stress. Biochim Biophys Acta 2004;1670:165-71.
- Bae EA, Han MJ, Baek NI, et al. In vitro anti-Helicobacter pylori activity of panaxytriol isolated from ginseng. Arch Pharm Res 2001;24:297-9. [PubMed]
- Park EK, Choo MK, Han MJ, et al. Ginsenoside Rh1 possesses antiallergic and anti-inflammatory activities. Int Arch Allergy Immunol 2004;133:113-20. [PubMed]
- Smolinski AT, Pestka JJ. Modulation of lipopolysaccharide-induced proinflammatory cytokine production in vitro and in vivo by the herbal constituents apigenin (chamomile), ginsenoside Rb(1) (ginseng) and parthenolide (feverfew). Food Chem Toxicol 2003;41:1381-90. [PubMed]
- Belogortseva NI, Yoon JY, Kim KH. Inhibition of Helicobacter pylori hemagglutination by polysaccharide fractions from roots of Panax ginseng. Planta Med 2000;66:217-20. [PubMed]
- Yoo SH, Jung HS, Sohn WS, et al. Volatile sulfur compounds as a predictor for esophagogastroduodenal mucosal injury. Gut Liver 2008;2:113-8. [PubMed]
- Choi K, Lee S, Lee J, et al. Beneficial Effect of Korea Red Ginseng on Halitosis; Attenuation of H2S Induced Inflammatory Mediators and cystathionine γ-lyase Expression. J Ginseng Res 2009;367-77.
- Jeong JH, Jeong HR, Jo YN, et al. Ameliorating effects of aged garlic extracts against Abeta-induced neurotoxicity and cognitive impairment. BMC Complement Altern Med 2013;13:268. [PubMed]
- Rana SV, Pal R, Vaiphei K, et al. Garlic in health and disease. Nutr Res Rev 2011;24:60-71. [PubMed]
- Guercio V, Galeone C, Turati F, et al. Gastric cancer and allium vegetable intake: a critical review of the experimental and epidemiologic evidence. Nutr Cancer 2014;66:757-73. [PubMed]
- Lee SY, Shin YW, Hahm KB. Phytoceuticals: mighty but ignored weapons against Helicobacter pylori infection. J Dig Dis 2008;9:129-39. [PubMed]
- Cañizares P, Gracia I, Gomez LA, et al. Optimization of Allium sativum solvent extraction for the inhibition of in vitro growth of Helicobacter pylori. Biotechnol Prog 2002;18:1227-32. [PubMed]
- O'Gara EA, Maslin DJ, Nevill AM, et al. The effect of simulated gastric environments on the anti-Helicobacter activity of garlic oil. J Appl Microbiol 2008;104:1324-31. [PubMed]
- O'Gara EA, Hill DJ, Maslin DJ. Activities of garlic oil, garlic powder, and their diallyl constituents against Helicobacter pylori. Appl Environ Microbiol 2000;66:2269-73. [PubMed]
- Cañizares P, Gracia I, Gomez LA, et al. Allyl-thiosulfinates, the bacteriostatic compounds of garlic against Helicobacter pylori. Biotechnol Prog 2004;20:397-401. [PubMed]
- Iimuro M, Shibata H, Kawamori T, et al. Suppressive effects of garlic extract on Helicobacter pylori-induced gastritis in Mongolian gerbils. Cancer Lett 2002;187:61-8. [PubMed]
- Gail MH, You WC. A factorial trial including garlic supplements assesses effect in reducing precancerous gastric lesions. J Nutr 2006;136:813S-5S. [PubMed]
- Gail MH, Pfeiffer RM, Brown LM, et al. Garlic, vitamin, and antibiotic treatment for Helicobacter pylori: a randomized factorial controlled trial. Helicobacter 2007;12:575-8. [PubMed]
- Fani A, Fani I, Delavar M, et al. Combined garlic-omeprazole versus standard quadruple therapy for eradication of Helicobacter pylori infection. Indian J Gastroenterol 2007;26:145-6. [PubMed]
- Zhang MJ, Spite M. Resolvins: anti-inflammatory and proresolving mediators derived from omega-3 polyunsaturated fatty acids. Annu Rev Nutr 2012;32:203-27. [PubMed]
- Harris WS. Achieving optimal n-3 fatty acid status: the vegetarian's challenge . . . or not. Am J Clin Nutr 2014;100:449S-52S. [PubMed]
- Serhan CN. Pro-resolving lipid mediators are leads for resolution physiology. Nature 2014;510:92-101. [PubMed]
- Siddiqui RA, Harvey KA, Zaloga GP, et al. Modulation of lipid rafts by Omega-3 fatty acids in inflammation and cancer: implications for use of lipids during nutrition support. Nutr Clin Pract 2007;22:74-88. [PubMed]
- Hutton ML, Kaparakis-Liaskos M, Turner L, et al. Helicobacter pylori exploits cholesterol-rich microdomains for induction of NF-kappaB-dependent responses and peptidoglycan delivery in epithelial cells. Infect Immun 2010;78:4523-31. [PubMed]
- Correia M, Michel V, Matos AA, et al. Docosahexaenoic acid inhibits Helicobacter pylori growth in vitro and mice gastric mucosa colonization. PLoS One 2012;7:e35072. [PubMed]
- Meier R, Wettstein A, Drewe J, et al. Fish oil (Eicosapen) is less effective than metronidazole, in combination with pantoprazole and clarithromycin, for Helicobacter pylori eradication. Aliment Pharmacol Ther 2001;15:851-5. [PubMed]
- Kuriki K, Wakai K, Matsuo K, et al. Gastric cancer risk and erythrocyte composition of docosahexaenoic acid with anti-inflammatory effects. Cancer Epidemiol Biomarkers Prev 2007;16:2406-15. [PubMed]
- Kim BG, Shin KS, Yoon TJ, et al. Fermentation of Korean red ginseng by Lactobacillus plantarum M-2 and its immunological activities. Appl Biochem Biotechnol 2011;165:1107-19. [PubMed]
- Chiu YH, Lu YC, Ou CC, et al. Lactobacillus plantarum MYL26 induces endotoxin tolerance phenotype in Caco-2 cells. BMC Microbiol 2013;13:190. [PubMed]
- Messier C, Epifano F, Genovese S, et al. Licorice and its potential beneficial effects in common oro-dental diseases. Oral Dis 2012;18:32-9. [PubMed]
- Penner R, Fedorak RN, Madsen KL. Probiotics and nutraceuticals: non-medicinal treatments of gastrointestinal diseases. Curr Opin Pharmacol 2005;5:596-603. [PubMed]
- Capurso G, Marignani M, Piciucchi M, et al. Probiotics and severe acute pancreatitis. J Clin Gastroenterol 2008;42 Suppl 3 Pt 1:S148-51. [PubMed]
- Petrof EO, Kojima K, Ropeleski MJ, et al. Probiotics inhibit nuclear factor-kappaB and induce heat shock proteins in colonic epithelial cells through proteasome inhibition. Gastroenterology 2004;127:1474-87. [PubMed]
- Kruis W, Fric P, Pokrotnieks J, et al. Maintaining remission of ulcerative colitis with the probiotic Escherichia coli Nissle 1917 is as effective as with standard mesalazine. Gut 2004;53:1617-23. [PubMed]
- Patel A, Shah N, Prajapati JB. Clinical appliance of probiotics in the treatment of Helicobacter pylori infection-A brief review. J Microbiol Immunol Infect 2014;47:429-37. [PubMed]
- Kim MN, Kim N, Lee SH, et al. The effects of probiotics on PPI-triple therapy for Helicobacter pylori eradication. Helicobacter 2008;13:261-8. [PubMed]
- Franceschi F, Cazzato A, Nista EC, et al. Role of probiotics in patients with Helicobacter pylori infection. Helicobacter 2007;12 Suppl 2:59-63. [PubMed]
- Gotteland M, Brunser O, Cruchet S. Systematic review: are probiotics useful in controlling gastric colonization by Helicobacter pylori? Aliment Pharmacol Ther 2006;23:1077-86. [PubMed]
- Ryan KA, O'Hara AM, van Pijkeren JP, et al. Lactobacillus salivarius modulates cytokine induction and virulence factor gene expression in Helicobacter pylori. J Med Microbiol 2009;58:996-1005. [PubMed]
- Lee JS, Paek NS, Kwon OS, et al. Anti-inflammatory actions of probiotics through activating suppressor of cytokine signaling (SOCS) expression and signaling in Helicobacter pylori infection: a novel mechanism. J Gastroenterol Hepatol 2010;25:194-202. [PubMed]
- Yang YJ, Chuang CC, Yang HB, et al. Lactobacillus acidophilus ameliorates H. pylori-induced gastric inflammation by inactivating the Smad7 and NFkappaB pathways. BMC Microbiol 2012;12:38. [PubMed]
- Wilhelm SM, Johnson JL, Kale-Pradhan PB. Treating bugs with bugs: the role of probiotics as adjunctive therapy for Helicobacter pylori. Ann Pharmacother 2011;45:960-6. [PubMed]
- Tong JL, Ran ZH, Shen J, et al. Meta-analysis: the effect of supplementation with probiotics on eradication rates and adverse events during Helicobacter pylori eradication therapy. Aliment Pharmacol Ther 2007;25:155-68. [PubMed]
- Vítor JM, Vale FF. Alternative therapies for Helicobacter pylori: probiotics and phytomedicine. FEMS Immunol Med Microbiol 2011;63:153-64. [PubMed]
- Bhatt A. Evolution of clinical research: a history before and beyond james lind. Perspect Clin Res 2010;1:6-10. [PubMed]


