The multiple facets of alpha-1-antitrypsin
Introduction
Alpha-1-antitrypsin (AAT) was first identified as an inhibitor of the proteinase trypsin (and hence its early name) in the 1950’s (1). Its’ importance emerged following the description of five blood samples in which the α1 protein band was weak to absent on paper electrophoresis. Clinical assessment showed that three of the patients identified with this abnormality had severe, early onset of pulmonary emphysema (2), a condition that generally presents in late middle life. Subsequent family studies confirmed the association with chronic lung disease and the hereditary nature of the α1 protein deficiency.
These observations were described at the same time as an animal model of emphysema induced by the plant proteinase papain (3). These two independent observations led to the assumption that an enzyme or enzymes, normally controlled by AAT, was responsible for a tissue breakdown process in the lung that resulted in destruction of alveolar integrity resulting in emphysematous change.
Molecular studies indicated that AAT was not only an inhibitor of serine proteinases but that its inhibitory function and specificity was limited to an inhibitory loop and a critical amino acid at position 342 near the carboxy terminus. In the native protein this is methionine and leads to it being most active against human neutrophil elastase (NE) (4). This critical methionine residue can be readily oxidised leading to major reduction in its ability to inhibit proteinases like elastase (4). Interestingly a natural variant in which the active site methionine is replaced by arginine converts AAT into an inhibitor of thrombin resulting in a spontaneous bleeding diathesis when an acute phase response increases the concentration (5). Alternatively active site mutation replacing the methionine with valine can lead to an oxidant resistant form of elastase inhibitor (4).
As these studies were underway purification of purified elastase from the neutrophil (6) and subsequently one of the other neutrophilic serine proteinases, proteinase 3 (7) were shown to produce emphysema like lesions in animal models. These observations led to the proteinase/antiproteinase theory of the development of emphysema [later applied to the more generic clinical syndrome of chronic obstructive pulmonary disease (COPD)]. The concept was simple, in that serine proteinases were the mediators of the tissue damage in emphysema. In AAT deficiency this occurred because of a lack of tissue protection by the inhibitor. However an alternative in subjects with normal AAT levels was that an overwhelming and excessive release of the cognate enzyme overcame the normal inhibitory protection, providing two mechanisms of disruption to the proteinase/proteinase balance required for health (8). A third alternative proposed was that the active site methionine was oxidised by cigarette smoke producing a localised functional deficiency.
Indeed two early studies of AAT in lavage fluid from smokers confirmed a reduction in function (9,10). These studies formed the basis of an entrenched concept to explain a tip in the proteinase/anti proteinase balance in the lung and other inflamed sites like the gingival cleft (11). Nevertheless this observation failed to explain why only a proportion of smokers developed COPD as it reflects (at best) a simple chemical interaction that should affect all.
However subsequent studies failed to confirm these observations showing no difference in AAT function in smokers (12-14) and indeed no significant evidence of oxidised methionine in the lung (15). Thus the simple pathway of release of serine proteinases by neutrophils in the lung leading to lung damage when AAT was quantitatively or functionally deficient failed to solve the pathophysiological processes that lead to the development and progression of COPD and emphysema.
Meanwhile other classes of enzymes such as the metalloproteinases (16) and the cysteine proteinase cathepsin B (17) have been implicated as causative in the development of COPD. In particular the former class of enzymes has been implicated in airflow obstruction due to damage of the small airways (18) and the latter in the development of emphysema. Since both processes are a feature of the lungs of patients with both centrilobular and the panlobular emphysema, more typical of AAT deficient subjects (19), the link to AAT and its deficiency seems more tenuous. However it is now apparent that there is a complex cascade of interacting proteinases and inhibitors whereby neutrophilic proteinases active precursors of other proteinase classes whilst inactivating their inhibitors leading to amplification which may add to the cycle by inactivating AAT thereby perpetuating the activity of the serine proteinases and so on.
As these concepts developed, the pro-inflammatory functions of proteolytic enzymes and the “anti-inflammatory effects” of their inhibitors resulted in a new paradigm exploring the anti-inflammatory properties of AAT.
Role of AAT in the inflammatory pathway
The generation, maintenance and resolution of inflammation is a complex sequence of events involving the release and balance between pro and anti-inflammatory cytokines and inflammatory cell infiltration.
The key effector cell in the pathophysiology of emphysema remains the neutrophil. The lungs of subjects with AAT deficiency demonstrate an increased neutrophil burden (20) in response to the chemoattractant interleukin 8 (CXCL8) and especially leukotriene B4 (21). Both of these chemoattractants can be produced by the action of NE on epithelial cells (22) and macrophages (23) respectively. Since neutrophils are the source of NE the presence of AAT deficiency would facilitate enzyme activity in the lung and hence chemoattractant production and release from these airways cells and thereby perpetuate neutrophil recruitment.
Indeed these cytokines (especially LTB4) are increased in the lungs of patients with AAT deficiency and inflammation is generally increased compared to non-deficient COPD (24). This difference is retained during episodes of acute infective exacerbations (25) and augmentation with regular infusions of AAT reduced the inflammation, LTB4 and the NE activity in the lung (26). Indeed such an approach has also been demonstrated in the inflamed lungs of non-deficient subjects with excessive neutrophilic infiltration and free NE activity such as cystic fibrosis (27) and pneumonia (28) where AAT inhalation leads to elastase inhibition and a reduction in the downstream effects of inflammation.
Another feature of inflammation is the associated oxidant stress which can not only lead to tissue damage but also stimulate a pro-inflammatory response (29). As indicated above methionine is a powerful antioxidant and AAT has four methionine residues. It is therefore possible that part of its efficacy in these inflamed tissues is the inactivation of some of the free oxygen species by AAT, although there are of course more specific molecules to fulfil this role. However, studies have shown that AAT also suppresses superoxide production by activated neutrophils (30) thereby potentially reducing oxidant driven amplification of inflammation.
A third factor in the inflammatory cascade is the activation and recruitment of inflammatory cells. AAT reduces neutrophil chemotaxis in vitro (31) which may well reflect its ability to inhibit cellular cathepsin G activity. This mechanism would modulate the recruitment of neutrophils and hence the downstream inflammatory response driven by these cells and their products.
A more generic anti-inflammatory role has been assessed by studying the effect of AAT on the production and release of TNFα. This pro-inflammatory cytokine has a multitude of functions including neutrophil activation, up regulation of adhesion molecules and stimulation of the production and release of other cytokines. In animal models AAT prevents the lethal effects of TNFα administered to mice (32) and TNFα induced apoptosis (33). In addition, studies have shown that although endothelial cells stimulated with TNFα increase TNFα gene expression in the presence of AAT, subsequent TNFα secretion was reduced, probably as a result of inactivation of cell membrane tumor necrosis factor alpha-converting enzyme (TACE) required to cleave pro TNFα releasing it into the supernatant (34).
In similar studies AAT also blocks TNFα (and other cytokines) release from monocytes and neutrophils (35) and lung epithelial cells (36) in response to endotoxin. In addition, more recent studies have examined the response of neutrophils from AAT deficient subjects and controls in vitro to exogenous TNFα in the absence and presence of AAT (34). The data showed AAT deficient neutrophils had an enhanced response to exogenous TNFα resulting in increased degranulation and TNFα production. Addition of AAT markedly inhibited these responses in the control cells but although the same trend was seen in AAT deficient cells this failed to achieve statistical significance over 20 minutes perhaps because of the increased baseline activation. Nevertheless, consistent anti TNFα effects of AAT have been verified by others (37,38).
The exact mechanism remains uncertain, although in the studies by Bergin et al. (39) the data suggested AAT blocked the TNFα receptor on the cell membrane. In many studies purified native or recombinant AAT has been used and this retains its proteinase inhibitory properties. Therefore these effects could potentially relate to an enzyme or enzymes unknown that mediate the inflammatory response and hence become inactivated in the presence of AAT. In this respect, the studies by Bergin et al. (39) also used antithrombin III as an alternative serine proteinase inhibitor (though with a difference spectrum of enzyme inhibitory activity) and found that unlike AAT it had no effect. However other studies have taken an alternative approach by using AAT that had been inactivated (at least as an elastase inhibitor) by oxidation of the active site (37) and showed the non-functional AAT to be equally effective in preventing TNFα responses. Perhaps the clearest example relates to the smoking mouse model. These animals have a TNFα response with downstream inflammation, proteinase release and tissue destruction leading to emphysematous change. Administration of oxidised AAT blocked all these effects thus acting at a high level in the inflammatory pathway (40). More recently, Jonigk and colleagues used a recombinant form of AAT linked to the IgG Fc fragment that also inactivates its anti-elastase properties. This form of AAT also abrogates endotoxin induced lung inflammation and TNFα release in mice using a combination of in vivo and in vitro neutrophil studies (41).
Thus AAT can have suppressive activity on the inflammatory pathway by both an enzyme inhibitory and a non inhibitory mechanism. The molecular domain responsible for the latter process remains unknown. These mechanisms and concepts are summarised in the Figure 1.
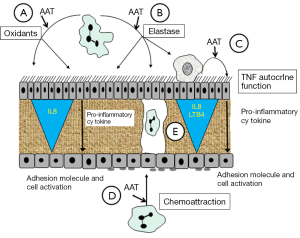
Immune modulation
In recent years the function of AAT in modulation of disease processes, not conventionally associated with genetic deficiency, has been explored especially diabetes and pancreatic cell transplantation. AAT prevents the development of overt hyperglycaemia in non obese diabetic mice (42) and increases insulin secretion and reduces cytokine mediated apoptosis of pancreatic B cells in vitro (43). Indeed the anti-apoptosis activity of AAT has also been suggested as a further mechanism whereby the development of emphysema via an apoptotic pathway could also be enhanced in AATD (44). However, in addition AAT prolongs autologous cell allograft survival in mice (45) by increasing immune tolerance (46). These data have led to exploration of potential immune mechanism in AAT deficiency. Certainly the increased incidence of vasculitis in patients with AATD is associated with anti neutrophilic cytoplasmic antigen (ANCA) auto-antibodies supporting a role of AAT in suppressing immunity, although of course this can also occur in non deficient subjects. Studies assessing other auto-antibodies with a potential role in the lung disease such as anti-elastin antibodies have been negative (47,48). However auto-immune hypothyroidism is also increased in AATD (49) suggesting that a reduction in immune tolerance may have a role outside the lung.
Finally, more recently pathological studies of lungs from AATD subjects have been compared to those from patients with usual COPD to determine if there was histological evidence of increased immune response (T & B cells and lymphoid follicles) in the deficient patients. Again, the data has proven to be negative (50), so this role, though of interest, needs further study to support or dismiss this potential function.
Other functions
Antimicrobial activity has been explored as a function of other proteinase inhibitors and AAT is no exception. AAT inhibits human HIV virus type 1 (51), reduces tonsillar B cell proliferation to moraxella catarrhalis (52) and suppresses pseudomonas proliferation in a model of lung infection (53). In addition AAT reduces ischaemia reperfusion injury by the prevention of apoptosis (54) a mechanism applicable to emphysema and diabetes as indicated above. Finally there is some data to indicate that AAT blocks the antigen and mediator induced airway responses in animal models (55) and elastase levels are increased in the sputum of asthmatic patients (56). Since neutrophil proteinases also activate eosinophils (57) and induce airway smooth muscle proliferation (58), it is possible an anti-inflammatory property may also have a role in asthma in humans. Further studies are clearly indicated.
Summary
The prime function of AAT remains its inhibitory function against serine proteinases protecting tissues from damage. Data suggests that this may play both a direct and indirect role in a proteolytic cascade. However in addition AAT may abrogate inflammation via both an enzyme inhibitory and non-inhibitory role. Whether AAT also plays an additional immune modulatory role in the lung seems unlikely but may be relevant in other diseases such as diabetes, hypothyroidism, vascular injury and perhaps asthma.
Acknowledgements
Disclosure: Grifols and CSL Behring provided non-commercial grant support and Kamada were responsible for clinical trial design and delivery.
References
- Jacobsson K. I. Studies on the determination of fibrinogen in human blood plasma. II. Studies on the trypsin and plasmin inhibitors in human blood serum. Scand J Clin Lab Invest 1955;7 Suppl. 14:3-102. [PubMed]
- Ganrot PO, Laurell CB, Eriksson S. Obstructive lung disease and trypsin inhibitor in alpha- 1-antitrypsin deficiency. Scand J Clin Lab Invest 1967;19:205-8. [PubMed]
- Gross P. Experimental Emphysema: Its production with papain in normal and silicotic rats. Arch Environ Health 1965;11:50-8. [PubMed]
- Johnson D, Travis J. The oxidative inactivation of human alpha-1-proteinase inhibitor. Further evidence for methionine at the reactive center. J Biol Chem 1979;254:4022-6. [PubMed]
- Owen MC, Brennan SO, Lewis JH, et al. Mutation of antitrypsin to antithrombin. alpha 1-antitrypsin Pittsburgh (358 Met leads to Arg), a fatal bleeding disorder. N Engl J Med 1983;309:694-8. [PubMed]
- Senior RM, Tegner H, Kuhn C, et al. The induction of pulmonary emphysema with human leukocyte elastase. Am Rev Respir Dis 1977;116:469-75. [PubMed]
- Kao RC, Wehner NG, Skubitz KM, et al. Proteinase 3. A distinct human polymorphonuclear leukocyte proteinase that produces emphysema in hamsters. J Clin Invest 1988;82:1963-73. [PubMed]
- Stockley RA. Proteases and antiproteases. In: Chadwick D and Goode JA, eds. chronic obstructive pulmonary disease: Pathogenesis to treatment. Chichester: John Wiley, 2001;189-204.
- Gadek JE, Fells GA, Crystal RG. Cigarette smoking induces functional antiprotease deficiency in the lower respiratory tract of humans. Science 1979;206:1315-6. [PubMed]
- Carp H, Miller F, Hoidal JR, et al. Potential mechanism of emphysema: alpha 1-proteinase inhibitor recovered from lungs of cigarette smokers contains oxidized methionine and has decreased elastase inhibitory capacity. Proc Natl Acad Sci U S A 1982;79:2041-5. [PubMed]
- Petropoulou P, Zhang Z, Curtis MA, et al. Measurement of both native and inactivated forms of alpha1 proteinase inhibitor in human inflammatory extracellular fluids. J Clin Periodontol 2003;30:795-801. [PubMed]
- Stone PJ, Calore JD, McGowan SE, et al. Functional alpha 1-protease inhibitor in the lower respiratory tract of cigarette smokers is not decreased. Science 1983;221:1187-9. [PubMed]
- Boudier C, Pelletier A, Gast A, et al. The elastase inhibitory capacity and the alpha 1-proteinase inhibitor and bronchial inhibitor content of bronchoalveolar lavage fluids from healthy subjects. Biol Chem Hoppe Seyler 1987;368:981-90. [PubMed]
- Burnett D, Afford SC, Campbell EJ, et al. Evidence for lipid-associated serine proteases and metalloproteases in human bronchoalveolar lavage fluid. Clin Sci (Lond) 1988;75:601-7. [PubMed]
- Morrison HM, Burnett D, Stockley RA. The effect of catalase and methionine-S-oxide reductase on oxidised alpha 1-proteinase inhibitor. Biol Chem Hoppe Seyler 1986;367:371-8. [PubMed]
- Hautamaki RD, Kobayashi DK, Senior RM, et al. Requirement for macrophage elastase for cigarette smoke-induced emphysema in mice. Science 1997;277:2002-4. [PubMed]
- Lesser M, Padilla ML, Cardozo C. Induction of emphysema in hamsters by intratracheal instillation of cathepsin B. Am Rev Respir Dis 1992;145:661-8. [PubMed]
- Churg A, Zhou S, Wright JL. Series "matrix metalloproteinases in lung health and disease": Matrix metalloproteinases in COPD. Eur Respir J 2012;39:197-209. [PubMed]
- McDonough JE, Yuan R, Suzuki M, et al. Small-airway obstruction and emphysema in chronic obstructive pulmonary disease. N Engl J Med 2011;365:1567-75. [PubMed]
- Morrison HM, Kramps JA, Burnett D, et al. Lung lavage fluid from patients with alpha 1-proteinase inhibitor deficiency or chronic obstructive bronchitis: anti-elastase function and cell profile. Clin Sci (Lond) 1987;72:373-81. [PubMed]
- Woolhouse IS, Bayley DL, Stockley RA. Sputum chemotactic activity in chronic obstructive pulmonary disease: effect of alpha(1)-antitrypsin deficiency and the role of leukotriene B(4) and interleukin 8. Thorax 2002;57:709-14. [PubMed]
- Walsh DE, Greene CM, Carroll TP, et al. Interleukin-8 up-regulation by neutrophil elastase is mediated by MyD88/IRAK/TRAF-6 in human bronchial epithelium. J Biol Chem 2001;276:35494-9. [PubMed]
- Hubbard RC, Fells G, Gadek J, et al. Neutrophil accumulation in the lung in alpha 1-antitrypsin deficiency. Spontaneous release of leukotriene B4 by alveolar macrophages. J Clin Invest 1991;88:891-7. [PubMed]
- Hill AT, Bayley DL, Campbell EJ, et al. Airways inflammation in chronic bronchitis: the effects of smoking and alpha1-antitrypsin deficiency. Eur Respir J 2000;15:886-90. [PubMed]
- Hill AT, Campbell EJ, Bayley DL, et al. Evidence for excessive bronchial inflammation during an acute exacerbation of chronic obstructive pulmonary disease in patients with alpha(1)-antitrypsin deficiency (PiZ). Am J Respir Crit Care Med 1999;160:1968-75. [PubMed]
- Stockley RA, Bayley DL, Unsal I, et al. The effect of augmentation therapy on bronchial inflammation in alpha1-antitrypsin deficiency. Am J Respir Crit Care Med 2002;165:1494-8. [PubMed]
- Griese M, Latzin P, Kappler M, et al. alpha1-Antitrypsin inhalation reduces airway inflammation in cystic fibrosis patients. Eur Respir J 2007;29:240-50. [PubMed]
- Geraghty P, Rogan MP, Greene CM, et al. Alpha-1-antitrypsin aerosolised augmentation abrogates neutrophil elastase-induced expression of cathepsin B and matrix metalloprotease 2 in vivo and in vitro. Thorax 2008;63:621-6. [PubMed]
- Rahman I, Adcock IM. Oxidative stress and redox regulation of lung inflammation in COPD. Eur Respir J 2006;28:219-42. [PubMed]
- Bucurenci N, Blake DR, Chidwick K, et al. Inhibition of neutrophil superoxide production by human plasma alpha 1-antitrypsin. FEBS Lett 1992;300:21-4. [PubMed]
- Lomas DA, Stone SR, Llewellyn-Jones C, et al. The control of neutrophil chemotaxis by inhibitors of cathepsin G and chymotrypsin. J Biol Chem 1995;270:23437-43. [PubMed]
- Libert C, Van Molle W, Brouckaert P, et al. alpha1-Antitrypsin inhibits the lethal response to TNF in mice. J Immunol 1996;157:5126-9. [PubMed]
- Van Molle W, Libert C, Fiers W, et al. Alpha 1-acid glycoprotein and alpha 1-antitrypsin inhibit TNF-induced but not anti-Fas-induced apoptosis of hepatocytes in mice. J Immunol 1997;159:3555-64. [PubMed]
- Bergin DA, Reeves EP, Meleady P, et al. α-1 Antitrypsin regulates human neutrophil chemotaxis induced by soluble immune complexes and IL-8. J Clin Invest 2010;120:4236-50. [PubMed]
- Nita I, Hollander C, Westin U, et al. Prolastin, a pharmaceutical preparation of purified human alpha1-antitrypsin, blocks endotoxin-mediated cytokine release. Respir Res 2005;6:12. [PubMed]
- Lockett AD, Kimani S, Ddungu G, et al. α1-Antitrypsin modulates lung endothelial cell inflammatory responses to TNF-α. Am J Respir Cell Mol Biol 2013;49:143-50. [PubMed]
- Subramaniyam D, Virtala R, Pawłowski K, et al. TNF-alpha-induced self expression in human lung endothelial cells is inhibited by native and oxidized alpha1-antitrypsin. Int J Biochem Cell Biol 2008;40:258-71. [PubMed]
- Janciauskiene S, Larsson S, Larsson P, et al. Inhibition of lipopolysaccharide-mediated human monocyte activation, in vitro, by alpha1-antitrypsin. Biochem Biophys Res Commun 2004;321:592-600. [PubMed]
- Bergin DA, Reeves EP, Hurley K, et al. The circulating proteinase inhibitor α-1 antitrypsin regulates neutrophil degranulation and autoimmunity. Sci Transl Med 2014;6:217ra1.
- Churg A, Wang RD, Xie C, et al. alpha-1-Antitrypsin ameliorates cigarette smoke-induced emphysema in the mouse. Am J Respir Crit Care Med 2003;168:199-207. [PubMed]
- Jonigk D, Al-Omari M, Maegel L, et al. Anti-inflammatory and immunomodulatory properties of α1-antitrypsin without inhibition of elastase. Proc Natl Acad Sci U S A 2013;110:15007-12. [PubMed]
- Lu Y, Tang M, Wasserfall C, et al. Alpha1-antitrypsin gene therapy modulates cellular immunity and efficiently prevents type 1 diabetes in nonobese diabetic mice. Hum Gene Ther 2006;17:625-34. [PubMed]
- Zhang B, Lu Y, Campbell-Thompson M, et al. Alpha1-antitrypsin protects beta-cells from apoptosis. Diabetes 2007;56:1316-23. [PubMed]
- Petrache I, Fijalkowska I, Zhen L, et al. A novel antiapoptotic role for alpha1-antitrypsin in the prevention of pulmonary emphysema. Am J Respir Crit Care Med 2006;173:1222-8. [PubMed]
- Lewis EC, Shapiro L, Bowers OJ, et al. Alpha1-antitrypsin monotherapy prolongs islet allograft survival in mice. Proc Natl Acad Sci U S A 2005;102:12153-8. [PubMed]
- Lewis EC, Mizrahi M, Toledano M, et al. alpha1-Antitrypsin monotherapy induces immune tolerance during islet allograft transplantation in mice. Proc Natl Acad Sci U S A 2008;105:16236-41. [PubMed]
- Greene CM, Low TB, O'Neill SJ, et al. Anti-proline-glycine-proline or antielastin autoantibodies are not evident in chronic inflammatory lung disease. Am J Respir Crit Care Med 2010;181:31-5. [PubMed]
- Wood AM, de Pablo P, Buckley CD, et al. Smoke exposure as a determinant of autoantibody titre in α1-antitrypsin deficiency and COPD. Eur Respir J 2011;37:32-8. [PubMed]
- Stone H, Pye A, Stockley RA. Disease associations in alpha-1-antitrypsin deficiency. Respir Med 2014;108:338-43. [PubMed]
- Baraldo S, Turato G, Lunardi F, et al. Immune activation in α1-antitrypsin-deficiency emphysema. Beyond the protease-antiprotease paradigm. Am J Respir Crit Care Med 2015;191:402-9. [PubMed]
- Shapiro L, Pott GB, Ralston AH. Alpha-1-antitrypsin inhibits human immunodeficiency virus type 1. FASEB J 2001;15:115-22. [PubMed]
- Hadzic R, Nita I, Tassidis H, et al. Alpha1-antitrypsin inhibits Moraxella catarrhalis MID protein-induced tonsillar B cell proliferation and IL-6 release. Immunol Lett 2006;102:141-7. [PubMed]
- Cantin AM, Woods DE. Aerosolized prolastin suppresses bacterial proliferation in a model of chronic Pseudomonas aeruginosa lung infection. Am J Respir Crit Care Med 1999;160:1130-5. [PubMed]
- Daemen MA, Heemskerk VH, van't Veer C, et al. Functional protection by acute phase proteins alpha(1)-acid glycoprotein and alpha(1)-antitrypsin against ischemia/reperfusion injury by preventing apoptosis and inflammation. Circulation 2000;102:1420-6. [PubMed]
- Forteza R, Botvinnikova Y, Ahmed A, et al. The interaction of alpha 1-proteinase inhibitor and tissue kallikrein in controlling allergic ovine airway hyperresponsiveness. Am J Respir Crit Care Med 1996;154:36-42. [PubMed]
- Vignola AM, Bonanno A, Mirabella A, et al. Increased levels of elastase and alpha1-antitrypsin in sputum of asthmatic patients. Am J Respir Crit Care Med 1998;157:505-11. [PubMed]
- Hiraguchi Y, Nagao M, Hosoki K, et al. Neutrophil Proteases Activate Eosinophil Function in vitro. Int Arch Allergy Immunol 2008;146:16-21. [PubMed]
- Huang CD, Chen HH, Wang CH, et al. Human neutrophil-derived elastase induces airway smooth muscle cell proliferation. Life Sci 2004;74:2479-92. [PubMed]


