Recent advances in the treatment of melanoma with BRAF and MEK inhibitors
Background
The incidence of melanoma has increased rapidly in recent decades, and approximately 10% of patients who are diagnosed with melanoma die from this cause (1). The sequence of events by which benign melanocytic nevi becomes melanoma is poorly understood but is believed to be due to progressive multiple genetic mutations that alter cell proliferation and differentiation.
Consistent with the results obtained in the different randomized meta-analysis over several years, the standard treatment in clinical trials for metastatic melanoma patients was the use of single-agent chemotherapy (dacarbazine, temozolomide or fotemustine) which offers a marginal clinical benefit (2-4). The discovery of high genetic heterogeneity present in melanomas has resulted in a significant change in the management of these patients, becoming increasingly important the development of targeted therapies against specific genetic aberrations among which are mutations in BRAF or in NRAS. In the development of such mutations is particularly important the mitogen activated protein kinase (MAPK) pathway which is activated in most melanomas and typically occurs after the development of initial mutations in BRAF or NRAS (5). These mutations can be identified both in benign melanocytic proliferations as in all stages of invasive or metastatic melanoma, with the frequency of 36-45% BRAF mutations in primary melanomas and 42-55% in metastatic melanomas.
The presence of a BRAF mutation in a patient with primary melanoma seems to have a relation with the time to disease relapse or overall survival (OS), and has also been associated with a worse prognosis compared to those patients who lack the mutation (6). However, with the use of BRAF and/or MEK inhibitors it has been observed that the use of these compounds neutralize the deleterious effect of the mutation in BRAF and these patients have a better prognosis than BRAF wild-type (WTBRAF) patients (7-10). One difficulty that arises with targeted treatment with drugs that inhibit BRAF or MEK is the appearance of primary or secondary resistance to these agents which typically occurs 6-8 months after initiation of treatment. Therefore, there is currently a great interest in determining these resistance mechanisms in order to be able to develop new therapeutic strategies to enhance disease control and to obtain better and more durable responses.
Most molecular alterations in melanoma involve the intracellular MAPK signaling pathway, also known as extracellular-related kinase (ERK). Under normal physiological conditions, this pathway is a key mediator for cell growth by the three isoforms of RAS GTPases (HRAS, KRAS and NRAS). When a ligand binds to its receptor on the plasma membrane Ras activity is stimulated, and a major effector of Ras is precisely the Ras family of Araf, Braf and Craf. Raf proteins turn signal through phosphorylation and activation of MEK kinase, which subsequently activates Erk. The complexity of this pathway increases with the great variability present in all the different components. Three subtypes of RAS (HRAS, NRAS and KRAS), three subtypes of RAF (ARAF, BRAF and CRAF), two subtypes of MEK (ME1 and MEK2), and two subtypes of ERK (ERK1 and ERK2) have been described and characterize; they act as genes encoding proteins with no redundant own functions and the different mechanisms of interaction between them generate a large panel of therapeutic options (11) (Figure 1).
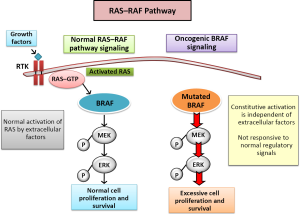
The activation of MAP kinase pathway by oncogenic mutations is described in 90% of melanomas. Hence therapies directed toward the MAP kinase pathway are an essential strategy to antagonize pathogenic effects of transduction pathways in melanomas. The Ras-Raf-Mek-Erk pathway is constitutively activated in tumors in humans through Ras or Raf. BRAF mutations are found in 8% of the tumors (50% in melanomas, 30-70% of thyroid cancer, 30% serous ovarian tumors of low grade and 10% of colorectal tumors) (12).
BRAF mutations in melanoma
There are many genetic alterations involved in the development and progression of melanoma and to date, more than 50 different mutations in the BRAF gene have been identified. Many of these mutations are located in the kinase domain with a kinase activity increased in Braf and Mek. Substituting a thymine for adenine in turn leading to the substitution of valine for glutamic acid (V600E) is the most common mutation in BRAF melanoma. This change causes a change in the kinase domain itself involving a permanent activation of B-RAF and by extension, of the MAPK pathway, which results in uncontrolled growth and proliferation of cells expressing v600EBRAF (13). Other less frequent mutations subtypes are BRAF V600K in 16% of melanoma patients and V600D in 3% of melanoma patients (14).
Despite the clear association of mutations in BRAF with the growth and development of malignant melanoma cells, such alterations have also been found in 80% of benign melanocytic nevi lesions (15) and do not exhibit degenerative changes to over the years, so it is believed that the presence of the mutation in melanoma v600EBRAF is not sufficient to activate the mechanisms of development of melanoma, and other genetic or epigenetic factors are also involved in the process (16).
Diagnostic strategies
Mutations in BRAF are normally examined by PCR sequencing. There are different methods of sequencing (Sanger, ABI BRAF), but the only one which is validated by the FDA is the Cobas 4800. It is more sensitive than other types of determinations such as Sanger or ABI method, and detects 99% of V600E mutations and 70% of V600K mutations (1).
NRAS mutations in melanoma
NRAS is the second most commonly activated oncogene in malignant melanomas after BRAF. Unlike what happens in BRAF mutations in benign nevus, NRAS mutations are only found in the 14% of melanoma samples. The presence of a mutation in NRAS in a patient with melanoma has its prognostic value. These patients usually have an age >55 years, have a pattern of chronic sun exposure, present at diagnosis in tumors with reduced thickness, increased number of mitoses and a lower frequency of ulceration, and the OS rate is less in these patients than in those with a mutations in BRAF (2).
Although mutations in BRAF and NRAS are mutually exclusive, there is evidence that a small fraction of patients with melanomas have coexisting clones with different oncogenic mutations. This mutational polyclonality has very important clinical implications to objectify the benefit obtained with the BRAF inhibitor therapy in patients with a mutation in NRAS. Although in several pre-clinical studies it has been evaluated the potential benefit of NRAS as a therapeutic agent, potent selective inhibitors of NRAS have not yet been developed to date and currently these patients are treated with chemotherapy or immunotherapy in the absence of treatment directed effective against this mutation (3-5).
BRAF inhibitors
After the discovery of the mutation in BRAF as a therapeutic target for those patients with metastatic melanoma, different BRAF inhibitor drugs have been tested in a clinical context with significant benefits in terms of increased response rate, increased progression-free survival (PFS) and increased OS in patients with the presence of a BRAF mutation (Table 1). Within the classification of these drugs, we highlight two groups of RAF inhibitor drugs: non-selective BRAF inhibitors and selective BRAF inhibitors.

Full table
Non-selective BRAF inhibitors
Sorafenib was the first BRAF inhibitor drug that was clinically developed in patients with metastatic melanoma. It has an activity directed toward multiple protein kinases as BRAF, CRAF, VEGF and PDGF in a non-selective manner so that its mechanism of inhibition has a wide range of possibilities (17).
Despite having been evaluated in several phases I, II and III studies as a single therapy or in combination with different chemotherapeutic agents, the clinical utility of sorafenib in melanoma has been proved to be very limited (18-21).
Selective BRAF inhibitors
Vemurafenib (PLX4032 and its analog PLX4720) is an oral drug which acts as a RAF inhibitor with a strong blockade activity against the v600EBRAF mutation. Vemurafenib was selected for clinical development vs. PLX4720 for its favorable pharmacokinetic characteristics. It inhibits the growth of cells that have mutations in v600EBRAF by blocking the activation of MAPK kinase pathway with subsequent cell senescence in the G1 phase of the cycle and apoptosis thereof. In vitro is also able to inhibit the proliferation of those cells expressing other melanoma mutations in the BRAF V600 such as v600DBRAF or BRAF V600K (6).
The clinical development of vemurafenib in patients suffering from metastatic melanoma has been set up forcefully through the development of different clinical trials. In a phase I study (BRIM-1) with vemurafenib given at a dose of 960 mg orally twice daily it was observed a tumor regression in 81% of patients with metastatic v600EBRAF mutation melanomas. After 15 days of treatment initiation, the different responses were associated with a decreased phospho-ERK (PERK), cyclin A D1 and ki-67 tumor levels as well as the inhibition of glucose uptake in some tumor lesions as measured by FDG-PET (8). A total of 49 patients with metastatic melanoma were included in the dose escalation phase and 32 additional patients with metastatic melanoma v600EBRAF were included in the expansion phase. In the cohort of dose escalation, among the 16 patients with melanoma v600EBRAF and receiving treatment at doses of 240 mg or more of PLX4032 twice daily, 10 patients had partial response and one patient had complete response. No clinical responses were observed in 5 patients with melanoma without mutation in BRAF. In the expansion cohort of 32 patients who had been treated at the recommended phase II of 960 mg twice daily dose study, 24 patients had a partial response and 2 patients had a complete response, so that the overall response rate was 81%. Survival at 1 and 2 years of diagnosis was 50% and 38%, respectively, with a median OS of 13.8 months (8). These findings stimulated the rapid recruitment of patients in both phase II study (BRIM-2) and the randomized phase III study (BRIM-3). In the phase II study, 132 patients with advanced melanoma which had received any prior systemic therapy were included. The 960 mg dose was administered orally twice daily. The objective response rate was 53% with a complete response rate of 5% and a median duration of response of 6.8 months. Disease progression was observed in 14% of patients and the median OS was 15.9 months. The most common side effects were grade 1 and 2 arthralgia, skin toxicity in the form of rash and photosensitivity, fatigue, and alopecia. The appearance of cutaneous squamous cell carcinomas occurred in 26% of patients included in the study (7).
Finally, in the pivotal phase III (BRIM-3) study 675 patients with metastatic melanoma v600EBRAF who previously had not received systemic treatment, were randomized to receive vemurafenib (960 mg orally twice daily) or chemotherapy with a scheme based on dacarbazine alone. The evaluation at 6 months of starting treatment showed an OS of 84% in the group of patients who had been treated with vemurafenib compared to 64% in the group receiving treatment with dacarbazine [hazard ratio (HR) =0.37; P<0.001]. The rate of death risk reduction was 63% and a reduced risk of death or disease progression of 74% compared to the group receiving treatment with dacarbazine was observed. The response rate to treatment was 48% in the group receiving vemurafenib compared to 5% in the group treated with dacarbazine. The median time to progression (TTP) was 5.3 months and 1.6 months for each patient group, respectively. Median OS, censored at crossover, was 13.6 months [95% confidence interval (CI), 12.0-15.2] in the vemurafenib group vs. 9·7 months (95% CI, 7.9-12.8) in the dacarbazine group; 12-month OS was 56% (95% CI, 50-61%) for vemurafenib and 44% (95% CI, 38-51%) for dacarbazine censored at crossover (Table 2) (8). Therefore, based on the results of the phases II and III trials in 2011 vemurafenib was approved by the Food and Drug Administration (FDA) and the European Medicines Agency (EMA) for those patients with unresectable or metastatic melanoma BRAF V600E mutation carriers (22).
Dabrafenib is another selective inhibitor of BRAF V600E and V600KBRAF mutations which has shown comparable results to those obtained with treatment with vemurafenib (9). In a phase I/II similar to that performed with PLX4032, 35 patients with metastatic melanoma and carriers of V600BRAF mutation were treated at two dose levels: 15 patients were treated at doses of 150 mg twice a day and 20 patients received treatment at a dose of 200 mg twice daily. In the interim analysis, 20 of 26 patients (77%) V600EBRAF mutation carriers had objective responses. In the second part of this study, they added an additional cohort of ten patients with brain disease which had not received any previous treatment. These patients were treated at doses of 150 mg twice daily; in the first interim analysis responses to treatment were seen in all patients in the study and three of them presenting complete response. The most common side effects reported were pyrexia (37%), fatigue (34%), nausea and vomiting (23%), headache (29%) and skin disorders (72%). The incidence of cutaneous squamous carcinoma was approximately seen in 15% of patients (9). In the phase II (BREAK-2) study, 92 patients with metastatic melanoma carriers of V600EBRAF and V600KBRAF mutation were included, observing a response rate of 59% in the cohort of patients with the mutation V600EBRAF and response in two patients with the mutation V600KBRAF. The median TTP compared to treatment with dacarbazine was 27 and 30 weeks, respectively (10). Finally, in the randomized phase III (BREAK-3) study, 250 patients with unresectable or metastatic V600EBRAF mutation melanoma carriers who had not received prior treatment were included. Patients were treated with a dose of dabrafenib 150 mg twice daily or dacarbazine at a dose of 1,000 mg/m2 every 3 weeks. In the pre-specified study analysis conducted in December 2011, dabrafenib reduced the relative risk of disease progression or death by 70% compared to treatment with dacarbazine (HR =0.30; 95% CI, 0.18-0.51; P<0.0001). Study data showed a median PFS of 5.1 months in the dabrafenib arm (95% CI, 4.9-6.9) vs. 2.7 months with dacarbazine treatment arm (95% CI, 1.5-3.2). In a subsequent analysis in June 2012, dabrafenib reduced the relative risk of disease progression or death by 63% compared to treatment with dacarbazine (HR =37; 95% CI, 0.24-0.58; P<0.0001). The data showed a median PFS of 6.9 months (95% CI, 5.2-9.0) compared to 2.7 months with dacarbazine treatment (95% CI, 1.5-3.2). A subsequent analysis of the data held in December 2012, showed an OS for dabrafenib at 12 months of enrollment of 70% compared with 63% observed for those patients treated with dacarbazine (HR =0.76; 95% CI, 0.48-1.21). Patients with melanoma with BRAF mutations other than V600EBRAF were excluded from the BREAK-3 trial; compared to patients with mutation V600KBRAF in single-arm studies, the activity appears to be lower than in V600EBRAF melanoma tumors (11,12). In the last up date of the BREAK-3 trial, it was observed an OS of 18.2 months in the dabrafenib arm and an OS of 15.2 months in the dacarbazine arm (12). A study with different quality-of-life analyses of the BREAK-3 study has been performed with a positive patient perception in the dabrafenib arm (Table 2) (13).

Full table
Both BRAF inhibitors (vemurafenib, dabrafenib) were also tested in patients with metastatic melanoma and disease in the brain, showing an apparent activity at the level of brain metastases despite the number of patients treated with vemurafenib is small to draw definitive conclusions compared to the number of patients with metastatic brain disease and who have been treated with dabrafenib (14-16).
Side effects related to treatment with inhibitors of BRAF
Generally BRAF inhibitors are well tolerated with few potentially serious side effects. The most common side effects include skin changes (50-70%, grades 1 and 2), fatigue (30-50%, grades 1, 2), diarrhea (10-30%, grades 1 and 2) and nausea (10-20%, grades 1 and 2). The major side effect associated with BRAF inhibitors is the development of cutaneous squamous cell carcinomas and keratoacanthomas (8). These lesions, which have a very fast growth, can be managed effectively by surgical removal without further evidence of recurrence. Its development has been associated with a paradoxical stimulation by the activation of the MAPK pathway on those healthy cells lacking BRAF alteration (BRAFWT) (23). Preclinical studies have also shown that adding treatment with a MEK inhibitor in conjunction with a BRAF inhibitor in patients with metastatic melanoma V600EBRAF mutation seems to diminish the proliferative effects caused by BRAF inhibition and a skin level it substantially reduces the incidence of squamous cell carcinoma in such patients (24).
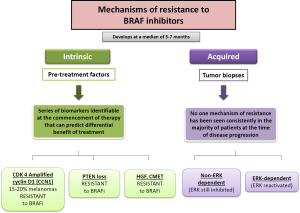
Mechanisms of resistance to BRAF: escape routes
BRAF inhibitors are very effective drugs for the treatment of those melanoma patients’ carriers of V600BRAF mutation. However their effectiveness is reduced to a limited duration, with a median PFS of 6 months. In turn, the rate of complete responses objectified to treatment with any of BRAF inhibitors is less than 5% and about 20% of patients do not have objective response to treatment despite being patients carrying mutations in BRAF. Resistance to such therapies may be due to a previous selection of the clonal cells which carry various activating mutations in oncogenes. Such alterations may lead to the activation of alternative arrangements which can generate resistance to progression and treatment schedule. The presence of resistance to BRAF inhibitors drugs can be of two types: either caused by the presence of mutations in melanoma cells before the onset of treatment (intrinsic resistance) or by the appearance of new mutations in melanoma cells exposed to treatment (acquired resistance) (Figure 2). Malignant melanoma cells, despite of both intrinsic and acquired resistance to BRAF inhibitors therapy, generate cells with uncontrolled growth. Over time, the over-growth of these cells causes predominant population of those cells resistant to treatment with BRAF inhibitors against the population of cells that were initially sensitive to such treatments.
Intrinsic resistance
About 15% of patients treated with BRAF inhibitors do not achieve tumor regression, because of intrinsic/primary mechanisms of resistance, and most patients who respond to therapy ultimately develop a mechanism of acquired/secondary resistance, leading to progressive disease. The intrinsic resistance to BRAF inhibitors has been demonstrated in several pre-clinical studies. In these studies it is objective that a proportion of melanoma cell lines carrying the mutation V600EBRAF have a different sensitivity to different cell growth inhibitory factors that generates the exposure to treatment with BRAF inhibitors (25,26). It is known that melanomas have a profile of mutations and alterations in several genes, amongst which are included MITF, AKT3, COT, cyclin D1, cyclin kinase CDK-2, CDK-4 and the retinoblastoma protein (Rb); however, for many of these conditions it is not known which mechanisms modulate the behavior of malignant melanoma cells with mutations in BRAF and their response to treatment with BRAF inhibitors. Intrinsic mechanisms of resistance to BRAF inhibitors include the best known amplification or overexpression of cyclin D1, alterations in PTEN tumor suppressor gene and different ways of interaction with the microenvironment such as HGF secretion by stromal cells with subsequent c-Met activation and reactivation of the MAP kinase pathway and the PI3K/AKT/mTOR pathway (17).
Acquired resistance
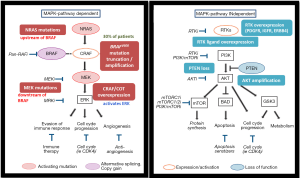
The reactivation of the MAPK pathway is the most frequent cause of acquired/secondary resistance. Contrary to what happens in the development of mechanisms of resistance to other targeted therapies in other tumor pathologies, it has not been described to date secondary mutations in the BRAF oncogene after sequencing multiple samples of malignant BRAF mutated melanoma exposed to treatment with BRAF inhibitors. Many of the mechanisms responsible for acquired resistance to BRAF inhibitors have been able to be reproduced in vitro after continuously expose of malignant melanoma cells carrying mutation V600EBRAF to different types of BRAF inhibitors. This resistance to BRAF inhibitors can be mediated via the reactivation of MAP kinase (MAP kinase-dependent) or by the activation of alternate signaling pathways such as PI3K/AKT (independent of MAP kinase) pathway (17) (Figure 3).
No association was observed between clinical outcome (best response and PFS) and specific mechanisms of resistance. Some tumors develop multiple mechanisms of resistance simultaneously in the same patient (intrapatient heterogeneity) or even in the same lesion (intratumor heterogeneity) (18,19). Within the mechanisms of resistance to BRAF inhibitors with acquired resistance dependent of the MAP kinase pathway there are the NRAS mutations, mutations in MEK 1/2, overexpression of COT, and reactivation of ERK after amplification and an alteration in the V600BRAF mutation. Finally, acquired resistance to BRAF inhibitors independent of the MAP kinase pathway activation comprises RKT, PTEN loss and amplification of AKT with increased survival and antiapoptotic signals after reactivation of the PI3K/AKT/mTOR (20,21).
MEK inhibitors
There is strong evidence that the mutation in BRAF is associated with selective activity and sensitivity to MEK inhibitor drugs. In the MAP kinase pathway, MEK is an effector in the signaling cascade of RAF. This dependence of MEK in the signaling pathway of RAF was determined in vitro in cells carrying a BRAF mutation. A deregulation in the expression of cyclin D1 was observed with consequent cellular senescence in the G1 phase of the cycle (27). Since both mutations in BRAF and NRAS are signaled by MEK 1 and 2, MEK inhibition appears to be an attractive therapeutic strategy for melanoma subtypes carriers of the mutation in BRAF and NRAS. Although the greatest clinical benefit (clinical response and/or survival benefit) has been objectified in those patients with mutations in BRAF, recent pre-clinical and clinical data also provide rationale for the potential use of MEK inhibitors in those patients affected metastatic melanoma and carriers of mutations in NRAS.
MEK inhibitors in BRAF or NRAS mutated melanomas
MEK inhibitors reduce proliferation, colony formation and invasiveness of melanoma cells carrying the mutation V600EBRAF both in vivo and in vitro (28). There are several MEK inhibitor drugs that have been tested in clinical trials for patients with metastatic or unresectable melanoma, among which there are selumetinib (AZD6244), PD-0325901, trametinib (GSK1120212), AS703026, cobimetinib (GDC-0973/XL518) and MEK162. To date, no MEK inhibitor has demonstrated clinical effectiveness such as objectified with BRAF inhibitors. Only the MEK inhibitors trametinib, cobimetinib and MEK 162 have shown in the clinical setting and in combination with BRAF inhibitors, increased activity in melanomas carriers of V600EBRAF mutations (29). In a phase III open-label trial 322 patients who had metastatic melanoma with a V600E or V600K BRAF mutation were randomly assigned to receive trametinib, an oral selective MEK inhibitor, or chemotherapy in a 2:1 ratio. Patients received trametinib (2 mg orally) once daily or intravenous dacarbazine (1,000 mg per square meter of body-surface area) or paclitaxel (175 mg per square meter) every 3 weeks. Patients in the chemotherapy group who had disease progression were permitted to cross over to receive trametinib. PFS was 4.8 months in the trametinib group and 1.5 months in the chemotherapy group (HR for disease progression or death in the trametinib group =0.45; 95% CI, 0.33-0.63; P<0.001). At 6 months, the rate of OS was 81% in the trametinib group and 67% in the chemotherapy group despite crossover (HR for death =0.54; 95% CI, 0.32-0.92; P=0.01). Rash, diarrhea, and peripheral edema were the most common toxic effects in the trametinib group and were managed with dose interruption and dose reduction; asymptomatic and reversible reduction in the cardiac ejection fraction and ocular toxic effects occurred infrequently. Secondary skin neoplasms were not observed (22). Trametinib, as compared with chemotherapy, improved rates of PFS and OS among patients who had metastatic melanoma with a BRAF V600E or V600K mutation although these results are not as good as those seen in the same population of patients which are treated with a BRAF inhibitor (29).
PD0325901 is a noncompetitive selective oral drug that inhibits ATP and the two isoforms of MEK (MEK 1/2). In a phase I clinical trial 48 patients with metastatic melanoma were included. Responses were observed in 3 patients, another 10 patients had stable disease for more than 4 months, and 15 patients showed reduction in tumor expression of Ki67 (30). However, side effects associated with the administration of the drug were rash and diarrhea which led to multiple drug dose reductions with consequent loss of inhibition of the MAPK kinase pathway (31).
AZD6244 or selumetinib is another potent, highly selective and reversible inhibitor of MEK 1 and 2. It was initially evaluated in a phase I clinical trial in which modest benefits were observed in a small subgroup of patients with metastatic melanoma mutation carriers of V600EBRAF (32). However, in the phase II randomized study the combination of selumetinib with dacarbazine vs. dacarbazine monotherapy in patients with melanoma V600EBRAF who had not received any previous treatment, tumor regression was observed in 12% of the patients and an increase in the median TTP of 5.6 vs. 3 months, respectively was observed. No differences in median OS between the two treatment groups (13.9 vs. 10.5 months, respectively) were observed (33). In another phase II randomized study with 200 patients with metastatic melanoma who had not received any previous treatment, it was evaluated the effect of administering AZD6244 treatment during cycles of 28 days compared to administer oral treatment with temozolomide. The TTP showed no statistically significant differences between groups. The objective response rate was 5.8% in patients who received treatment with AZD6244, and 9.4% in patients treated with temozolomide. Among those patients with the BRAF mutation, the objective response rate was also similar between groups (11.1% vs. 10.7%, respectively) (34). Finally, a pilot AZD6244 study in combination with dacarbazine, docetaxel or temsirolimus in 18 patients with advanced melanoma in which the mutational status of BRAF and NRAS was unknown, a response rate of 55% was observed in patients who were found to be BRAF mutated vs. a lack of response in patients who were not carriers of the BRAF mutation and in 4 patients with NRAS mutation. The median TTP was statistically significant in those patients with BRAF mutation in comparison with the group of patients without (8 vs. 2 months) (35).
Trametinib (GSK1120212) is an inhibitor of MEK 1 and 2, highly selective and reversible. The different phase I trials with this compound have demonstrated that the use of trametinib is safe and well tolerated with common side effects such as skin toxicity in the form of rash and diarrhea but which in turn are easy to operate with supportive treatment and close monitoring (36,37). In the first phase II study in patients with metastatic melanoma without determination of the mutational status of BRAF, the recommended daily dose of GSK1120212 was 2 mg. The dose limiting toxicities were skin toxicity in the form of rash, diarrhea and central venous retinopathy. In the 20 evaluable patients, 5 presented partial tumor responses with >50% reduction. In the 11 patients with BRAF mutation it was observed three partial responses, five disease stabilization and 3 progressions (35). In another phase II study with two cohorts of patients with metastatic melanoma with BRAF mutation, two groups were assigned: a group of patients who had previously received treatment with a BRAF inhibitor (cohort A, n=40), and another group of patients who had previously received chemotherapy or immunotherapy (cohort B, n=58). All study patients were treated with a dose of 2 mg GSK1120212 day. No objective responses were observed in cohort A and 28% of patients had stable disease; the median TTP was 1.8 months. In cohort B, a complete response, 23% partial response and 51% disease stabilization was observed; the median TTP was 4.0 months. The data obtained in this study suggested that treatment with trametinib is effective in those patients with BRAF mutation who have previously been treated with chemotherapy or immunotherapy, being ineffective the treatment with trametinib in patients who received frontline BRAF inhibitor treatment (38).
Finally, these results have been confirmed in a phase III trial in which patients with metastatic or unresectable V600E/KBRAF mutation melanoma were randomized to receive treatment with a dose of 2 mg trametinib daily or chemotherapy (paclitaxel or dacarbazine). The median TTP was 4.8 months in the subgroup who received treatment with trametinib compared with 1.5 months in the subgroup receiving chemotherapy (22). With 6 months of treatment, the OS rate was 81% in the group treated with trametinib compared with 67% in the group receiving chemotherapy.
Cobimetinib (GDC-0973/XL518) is a potent, highly selective inhibitor of MEK, also known as mitogen-activated protein kinase. In preclinical studies, oral dosing of cobimetinib resulted in potent and sustained inhibition of MEK in RAS- or BRAF-mutant tumor models (39).
MEK162 is the last 1 and 2 selective MEK inhibitor that has shown clinical activity. Preclinical studies have shown modest activity inhibiting the growth of tumor cells carrying NRAS or V600EBRAF mutation (40). The safety profile and preliminary evidence of tumor activity were previously reported in a phase I study in patients with advanced solid tumors. Later in the nonrandomized phase II study metastatic melanoma patients with NRAS or V600EBRAF mutation were included. Study patients were assigned to three treatment arms: MEK162 at doses of 45 mg twice daily for patients with NRAS mutations, MEK162 a dose of 45 mg twice daily for patients with BRAF mutation and MEK162 at doses 60 mg twice daily for those patients with BRAF mutation. The data obtained to date from the study include those of patients who were treated with a dose of 45 mg MEK162 (30 patients with NRAS mutation and 41 patients with BRAF mutation). The median follow-up of the study was 3.3 months and it was observed that 20% of patients with NRAS mutation and 20% of BRAF mutation patients had a partial response. The most common side effects were peripheral acneiform dermatitis, rash, facial edema and ocular disorders and diarrhea (41).
Combination of BRAF and MEK inhibitors
BRAF inhibitors provide a response rate and a median TTP higher than the one observed with the MEK inhibitor therapy for patients with metastatic melanoma and who carry a V600BRAF mutation. The role of MEK inhibitors in the treatment of these patients which have already progressed to a BRAF inhibitor is modest, with an only 5% of objective response rate without a clear impact in terms of PFS or in terms of OS (32). However, MEK inhibitors have a crucial role in those patients with metastatic BRAF mutation melanoma in combination with BRAF inhibitors. Also, note that this combination significantly reduces the side effects that occur at skin level (including squamous carcinomas and keratoacanthomas) with the use of BRAF inhibitors alone (42).
Studies evaluating the combination of a BRAF and a MEK inhibitor
Initial preclinical data demonstrated that the combination of MEK and BRAF inhibitors presents a superior synergistic activity in those tumor cells carrying the mutation in BRAF than either those components alone. The rationale for the combination of two drugs is based on the activity demonstrated in a pre-clinical level, the need to reverse the resistance observed with each drug alone and finally the advantage to reduce the incidence of hyperproliferative skin lesions which was objectified with the treatment with BRAF inhibitors (Table 2).
In a phase I/II study, 44 patients were treated with GSK212 (2 mg daily) + GSK436 (150 mg twice daily). Of the 16 evaluable patients, 13 patients had a partial response and 3 patients had stable disease. The objective response rate of all study patients was 81%. As for the reported side effects, 11% of the patients had pyrexia, 4% vomiting and 4% fatigue. No cases of squamous carcinoma or hyperproliferative lesions were observed in patients enrolled in the study. In the subsequent phase II randomized study for the combination of both drugs, 162 patients with metastatic melanoma V600E/KBRAF mutation were randomized to receive treatment with the combination of dabrafenib (150 mg) with trametinib (1 or 2 mg) or dabrafenib monotherapy (150 mg). The median follow-up time of the study was 14 months. The median TTP for the patient group receiving the combination and dose of 2 mg trametinib was 9.4 months compared to 5.8 months in the group treated with dabrafenib monotherapy (P<0.01). The response rate for trametinib 2 mg dose was 76% compared to 54% in the group receiving dabrafenib monotherapy (P=0.03), and OS at 12 months of the randomization was 78% for the dose of 2 mg trametinib compared with 70% in the group receiving dabrafenib alone. Typical hyperproliferative skin lesions related to BRAF inhibitor therapy decreased with the combination of both drugs (19% for the group treated with dabrafenib monotherapy vs. 7% for the combination of dabrafenib and trametinib) (43). In a phase III trial (COMBI-D), 423 previously untreated patients who had unresectable stage IIIC or stage IV melanoma with a V600EBRAF or V600KBRAF mutation were randomly assigned to receive a combination of dabrafenib (150 mg orally twice daily) and trametinib (2 mg orally once daily) or dabrafenib and placebo. The median PFS was 9.3 months in the dabrafenib-trametinib group and 8.8 months in the dabrafenib-only group (HR for progression or death in the dabrafenib-trametinib group =0.75; 95% CI, 0.57-0.99; P=0.03). The overall response rate was 67% in the dabrafenib-trametinib group and 51% in the dabrafenib-only group (P=0.002). At 6 months, the interim OS rate was 93% with dabrafenib–trametinib and 85% with dabrafenib alone (HR for death =0.63; 95% CI, 0.42-0.94; P=0.02). However, a specified efficacy-stopping boundary (two-sided P=0.00028) was not crossed. Rates of adverse events were similar in the two groups, although more dose modifications occurred in the dabrafenib-trametinib group. The rate of cutaneous squamous-cell carcinoma was lower in the dabrafenib-trametinib group than in the dabrafenib-only group (2% vs. 9%), whereas pyrexia occurred in more patients (51% vs. 28%) and was more often severe (grade 3, 6% vs. 2%) in the dabrafenib-trametinib group (44). In another phase III trial (COMBI-V) 704 patients with metastatic melanoma with a V600BRAF mutation were randomly assigned to receive either a combination of dabrafenib (150 mg twice daily) and trametinib (2 mg once daily) or vemurafenib (960 mg twice daily) orally as first-line therapy. At the preplanned interim OS analysis which was performed after 77% of the total number of expected events occurred, the OS rate at 12 months was 72% (95% CI, 67-77%) in the combination-therapy group and 65% (95% CI, 59-70%) in the vemurafenib group (HR for death in the combination-therapy group =0.69; 95% CI, 0.53-0.89; P=0.005). The pre-specified interim stopping boundary was crossed, and the study was stopped for efficacy in July 2014. Median PFS was 11.4 months in the combination therapy group and 7.3 months in the vemurafenib group (HR =0.56; 95% CI, 0.46-0.69; P<0.001). The objective response rate was 64% in the combination therapy group and 51% in the vemurafenib group (P<0.001). Rates of severe adverse events and study-drug discontinuations were similar in the two groups. Cutaneous squamous-cell carcinoma and keratoacanthoma occurred in 1% of patients in the combination-therapy group and 18% of those in the vemurafenib group (45).
Other studies have also explored in turn the efficiency of combined treatment of a BRAF inhibitor and a MEK inhibitor. In the phase Ib BRIM -7 study, the addition of cobimetinib to vemurafenib demonstrated an increased efficacy and decreased the skin toxicity seen with vemurafenib in monotherapy, in a statistically significant manner. The median TTP in the group of patients with a mutation in BRAF who had not received prior treatment with a BRAF inhibitor was 13.7 months compared to the 2.8 months seen in the group with a BRAF mutation and which had previously received treatment with a BRAF inhibitor. The rate of objectified response for the group that had not received prior treatment with a BRAF inhibitor was 85% compared to the 14% objectified in the group that had received previous treatment with a BRAF inhibitor. Most frequently reported toxicities were skin changes as rash (13%), liver disorders (19%) and diarrhea (8%) (46). In an initial phase Ib trial with the combination of vemurafenib and cobimetinib in patients with advanced V600BRAF mutated melanoma who had either recently progressed on vemurafenib or never received a BRAF inhibitor were included. In the dose-escalation phase, 129 patients received vemurafenib 720 or 960 mg twice a day continuously and cobimetinib 60, 80, or 100 mg once a day for 14 days on and 14 days off (14/14), 21 days on and 7 days off (21/7), or continuously (28/0). From the 129 total patients, 66 had recently progressed on vemurafenib and 63 had never received a BRAF inhibitor. The maximum tolerated dose was established as vemurafenib 960 mg twice a day in combination with cobimetinib 60 mg 21/7 days. Across all dosing regimens, the most common adverse events were diarrhea (83 patients, 64%), non-acneiform rash (77 patients, 60%), liver enzyme abnormalities (64 patients, 50%), fatigue (62 patients, 48%), nausea (58 patients, 45%), and photosensitivity (52 patients, 40%). Most adverse events were mild-to-moderate in severity. The most common grade 3 or 4 adverse events were cutaneous squamous-cell carcinoma (12 patients, 9%; all grade 3), raised amounts of alkaline phosphatase (11 patients, 9%), and anemia (9 patients, 7%). Confirmed objective responses were recorded in 10 (15%) of 66 patients who had recently progressed on vemurafenib, with a median PFS survival of 2·8 months (95% CI, 2.6-3.4). Confirmed objective responses were noted in 55 (87%) of 63 patients who had never received a BRAF inhibitor, including 6 (10%) who had a complete response; median PFS was 13.7 months (95% CI, 10.1-17.5) (46). Finally, in the phase III trial (COBRIM) 495 patients were randomly assigned to receive vemurafenib+ cobimetinib (60 mg QD, 21 days on/7 days off) or vemurafenib (960 mg BID) + placebo. Eligibility included treatment-naive V600BRAF mutation–positive patients with unresectable locally advanced or metastatic melanoma with adequate performance status and organ function. Median PFS was 9.9 months with the combination compared with 6.2 months with the control (HR =0.51; 95% CI, 0.39-0.68; P<0.0001). Objective response rate was 68% in the combination arm and 45% in the control arm (P<0.0001), including complete response in 10% in the combination arm and 4% of patients in the control group. Subgroup analyses of PFS based on key demographic and tumor characteristics were consistent with PFS in the intent-to-treat population, including those with normal or elevated baseline lactate dehydrogenase (LDH). PFS assessed by independent review was comparable with investigator-assessed PFS. Interim OS data showed an HR of 0.65 (95% CI, 0.42-1.00) but did not cross the pre-specified stopping boundary. Compared with vemurafenib alone, the combination was associated with a higher incidence of grade 3 or 4 adverse events (65% vs. 59%), with no difference in the rate of adverse events leading to study drug discontinuation (13% vs. 12%). Most grade ≥3 events occurred in the first 28 days and resolved quickly. Known MEK inhibitor-related toxicities such as diarrhea, serous retinopathy, elevated creatine phosphokinase, and increased liver transaminase levels were more commonly observed with the combination. The majority was grades 1 or 2, occurred between 1 and 4 months in the treatment course, and resolved quickly. The occurrence of secondary cutaneous neoplasms decreased with the combination (4% vs. 18%). Photosensitivity was more common in patients treated with the combination (all grades 32% vs. 18%) (47).
Long term survival with BRAF and MEK inhibitors
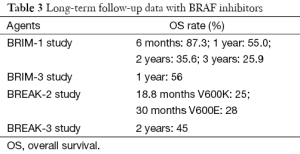
Long term data on BRAF inhibitor survival rates have been limited until recently when several results with monotherapy vemurafenib or dabrafenib are emerging (Table 3). In the BRIM-1 trial with vemurafenib monotherapy with a extension cohort of metastatic melanoma of 32 patients, median OS was 12.6 months. The OS rate at 6 months was of 87.3%, at 1 year of 55%, at 2 years of 35.6% and finally at 3 years of 25.9% (48). Extended follow-up analysis of vemurafenib in the phase III trial BRIM-3 has already been published. Median OS was initially not reported in the initial median follow-up time of 3.8 months but in the extended analysis, median OS, censored at crossover, was 13.6 months (95% CI, 12.0-15.2) in the vemurafenib group vs. 9.7 months (95% CI, 7.9-12.8) in the dacarbazine group; 12-month OS was 56% (95% CI, 50-61%) for vemurafenib and 44% (95% CI, 38-51%) for dacarbazine censored at crossover (49). In the phase II/III studies with dabrafenib, updated data has also been reported. In the phase II trial BREAK-2 with dabrafenib monotherapy in previously treated metastatic melanoma, OS was initially reported of 11.9 months; with a follow up of 13 months, median OS was 13.1 months. In the V600EBRAF group, 21 patients (28%) were alive beyond 30 months and in the V600KBRAF group, 3 patients (25%) were alive beyond 18.8 months. As for December 2013, 8 (9%) patients continued on dabrafenib treatment without disease progression with a median exposure of 37 months. In the phase III trial BREAK-3 the initial median OS was 18.2 months in the dabrafenib arm and 15.6 months in the dacarbazine arm; an update on OS and follow-up at 24 months was presented with 45% of dabrafenib and 32% of dacarbazine patients alive. Median OS in the dabrafenib arm was 20 vs. 15.6 months in the dacarbazine arm (HR =0.77, not statistically significant). 59% of dacarbazine patients crossed over to dabrafenib after confirmed progression. Long-term responders on dabrafenib were observed to have smaller lesions at baseline vs. patients that experienced disease progression. Maturing survival data suggest that a durable response is possible with dabrafenib in a subset of patients with no new signals identified with long-term treatment with dabrafenib. Data from the phase III trials with the combination of a BRAF and a MEK inhibitor vs. monotherapy with a BRAF inhibitor (COMBI-D, COMBI-V, coBRIM studies) in untreated patients with metastatic melanoma with BRAF V600E or V600K mutations, show a reduction in the risk of progression with an improvement in response rate. OS was longer in the combination arm, without increased overall toxicity (44,45,47).
Full table
Clinical evidence combining BRAF and MEK inhibition in patients with BRAFV600-mutant melanoma experiencing progression with single-agent BRAF inhibitor
In an open-label phase I/II study, patients treated with combination therapy after disease progression with a BRAF inhibitor treatment administered before study enrollment (part B; n=26) or after cross-over at progression with dabrafenib monotherapy (part C; n=45) were included. In parts B and C, confirmed objective response rates were 15% (95% CI, 4-35%) and 13% (95% CI, 5-27%), respectively; additionally, 50% and 44% of the patients experienced stable disease for 8 weeks, respectively. In part C, median PFS was 3.6 months (95% CI, 2-4), and median OS was 11.8 months (95% CI, 8-25) from cross-over. Patients who previously received dabrafenib for at least 6 months had superior outcomes with the combination treatment compared with those patients treated with dabrafenib for less than 6 months; median PFS was 3.9 (95% CI, 3-7) vs. 1.8 months (95% CI, 2-4), respectively (HR =0.49; P=0.02). Overall response rate was 26% (95% CI, 10-48%) vs. 0% (95% CI, 0-15%), respectively (50). In the phase Ib trial with vemurafenib and cobimetinib in BRAF mutant melanoma patients who had already progressed to vemurafenib, confirmed objective responses were recorded in 10 (15%) of 66 patients who had recently progressed on vemurafenib, with a median PFS of 2.8 months (95% CI, 2.6-3.4) (46).
Due to these results, it is concluded that the combination of a BRAF and MEK inhibitor has modest clinical efficacy in patients with BRAF inhibitor-resistant melanoma and this regimen may be a therapeutic strategy for patients who previously benefited from BRAF inhibitor monotherapy for more than 6 months but demonstrates minimal efficacy after rapid progression with previous BRAF inhibitor therapy.
Treatment of brain metastases in patients carrying the mutation in BRAF
Brain metastases are described in 10-40% of patients with metastatic melanoma and in a direct manner associated with a worse prognosis (43). It is estimated that approximately 20% of patients with metastatic melanoma have cerebral involvement at diagnosis of the disease. Systemic chemotherapy mainly based on alkylating agents such as temozolomide has a limited role in monitoring lesions in the central nervous system, with a response rate of <10% and a median survival of 3-5 months.
Dabrafenib has demonstrated activity in asymptomatic patients with metastasis which affect the central nervous system level. In a phase II study (BREAK-MB), patients with metastatic melanoma with disease at central nervous system and mutation carriers 600Glu Val and 600LysVal were included and distributed in two treatment groups based on the treatment they had previously received. It was observed a brain response rate of 39% in the group who had not received prior treatment, and a response rate of 31% in the group who had received previous treatment. The median TTP observed was 4 months and the median OS was nearly 8 months in both groups of patients. More responses were reported in the group of patients with the mutation 600LysVal. As for side effects, of the 172 patients included, 10 patients presented intracranial hemorrhage but only one of them was directly related to study treatment, and one patient had a seizure that was not related to the study treatment (16,51).
Vemurafenib has also shown clinical activity in patients with metastatic melanoma with brain metastases which are carriers of a V600BRAF mutation. In a multicenter phase II study, 24 patients with metastatic melanoma and symptomatic disease in the brain were included. They were treated with vemurafenib at a dose of 960 mg twice daily. A rate of intra- and extra cranial response of 42% and a response rate of cranial level >30% in 37% of the patients was observed. The median TTP was 3.9 months and median OS was 5.3 months. Regarding adverse effects, four cases of cutaneous squamous cell carcinoma were reported (45). The efficacy of vemurafenib in combination with cranial radiation was evaluated in a retrospective study of 12 patients with metastatic melanoma. After treatment, 64% of patients showed improvement of neurological symptoms and responses were observed in 75% of patients (48% complete response, 27% partial response) (52,53).
Conclusions
For many years, the treatment of metastatic melanoma had few therapeutic options and a lower OS rate. This scenario changed dramatically after the discovery of the mutation in BRAF in approximately 50% of patients with melanoma and further development of targeted therapies (against BRAF and MEK). However, and as happens in other diseases, these treatments have shown a benefit in OS, with rapid clinical responses, but with the appearance of early strength resistances. These findings emphasize the need to molecularly characterize the processes related to these resistance mechanisms in order to develop combinations of different inhibitors of MAP kinase pathway in an attempt to reverse this resistance. That is why it is a priority to analyze the data obtained in pre-clinical studies and design clinical studies, in order to observe prospectively in the form of serial biopsies, which mechanisms of resistance appear in these heterogeneous tumors in order better to select the best treatment for each patient, avoiding unnecessary costs and toxicities.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Heideman DA, Lurkin I, Doeleman M, et al. KRAS and BRAF mutation analysis in routine molecular diagnostics: comparison of three testing methods on formalin-fixed, paraffin-embedded tumor-derived DNA. J Mol Diagn 2012;14:247-55. [PubMed]
- Devitt B, Liu W, Salemi R, et al. Clinical outcome and pathological features associated with NRAS mutation in cutaneous melanoma. Pigment Cell Melanoma Res 2011;24:666-72. [PubMed]
- Eskandarpour M, Huang F, Reeves KA, et al. Oncogenic NRAS has multiple effects on the malignant phenotype of human melanoma cells cultured in vitro. Int J Cancer 2009;124:16-26. [PubMed]
- Eskandarpour M, Kiaii S, Zhu C, et al. Suppression of oncogenic NRAS by RNA interference induces apoptosis of human melanoma cells. Int J Cancer 2005;115:65-73. [PubMed]
- Glitza IC, Davies MA. Genotyping of cutaneous melanoma. Chin Clin Oncol 2014;3:27. [PubMed]
- Halaban R, Zhang W, Bacchiocchi A, et al. PLX4032, a selective BRAF(V600E) kinase inhibitor, activates the ERK pathway and enhances cell migration and proliferation of BRAF melanoma cells. Pigment Cell Melanoma Res 2010;23:190-200. [PubMed]
- Sosman JA, Kim KB, Schuchter L, et al. Survival in BRAF V600-mutant advanced melanoma treated with vemurafenib. N Engl J Med 2012;366:707-14. [PubMed]
- Chapman PB, Hauschild A, Robert C, et al. Improved survival with vemurafenib in melanoma with BRAF V600E mutation. N Engl J Med 2011;364:2507-16. [PubMed]
- Falchook GS, Long GV, Kurzrock R, et al. Dabrafenib in patients with melanoma, untreated brain metastases, and other solid tumours: a phase 1 dose-escalation trial. Lancet 2012;379:1893-901. [PubMed]
- Ascierto PA, Minor D, Ribas A, et al. Phase II trial (BREAK-2) of the BRAF inhibitor dabrafenib (GSK2118436) in patients with metastatic melanoma. J Clin Oncol 2013;31:3205-11. [PubMed]
- Espinosa E, Grob JJ, Dummer R, et al. Treatment algorithms in stage IV melanoma. Am J Ther 2015;22:61-7. [PubMed]
- Hauschild A, Grob JJ, Demidov LV, et al. Dabrafenib in BRAF-mutated metastatic melanoma: a multicentre, open-label, phase 3 randomised controlled trial. Lancet 2012;380:358-65. [PubMed]
- Grob JJ, Amonkar MM, Martin-Algarra S, et al. Patient perception of the benefit of a BRAF inhibitor in metastatic melanoma: quality-of-life analyses of the BREAK-3 study comparing dabrafenib with dacarbazine. Ann Oncol 2014;25:1428-36. [PubMed]
- Lin NU. Targeted therapies in brain metastases. Curr Treat Options Neurol 2014;16:276. [PubMed]
- Long GV, Margolin KA. Multidisciplinary approach to brain metastasis from melanoma: the emerging role of systemic therapies. Am Soc Clin Oncol Educ Book 2013.393-8. [PubMed]
- Long GV, Trefzer U, Davies MA, et al. Dabrafenib in patients with Val600Glu or Val600Lys BRAF-mutant melanoma metastatic to the brain (BREAK-MB): a multicentre, open-label, phase 2 trial. Lancet Oncol 2012;13:1087-95. [PubMed]
- Spagnolo F, Ghiorzo P, Orgiano L, et al. BRAF-mutant melanoma: treatment approaches, resistance mechanisms, and diagnostic strategies. Onco Targets Ther 2015;8:157-68. [PubMed]
- Klein O, Clements A, Menzies AM, et al. BRAF inhibitor activity in V600R metastatic melanoma--response. Eur J Cancer 2013;49:1797-8. [PubMed]
- Shi H, Hugo W, Kong X, et al. Acquired resistance and clonal evolution in melanoma during BRAF inhibitor therapy. Cancer Discov 2014;4:80-93. [PubMed]
- Das Thakur M, Stuart DD. Molecular pathways: response and resistance to BRAF and MEK inhibitors in BRAF(V600E) tumors. Clin Cancer Res 2014;20:1074-80. [PubMed]
- Van Allen EM, Wagle N, Sucker A, et al. The genetic landscape of clinical resistance to RAF inhibition in metastatic melanoma. Cancer Discov 2014;4:94-109. [PubMed]
- Flaherty KT, Robert C, Hersey P, et al. Improved survival with MEK inhibition in BRAF-mutated melanoma. N Engl J Med 2012;367:107-14. [PubMed]
- Hertzman Johansson C, Egyhazi Brage S. BRAF inhibitors in cancer therapy. Pharmacol Ther 2014;142:176-82. [PubMed]
- Manousaridis I, Mavridou S, Goerdt S, et al. Cutaneous side effects of inhibitors of the RAS/RAF/MEK/ERK signalling pathway and their management. J Eur Acad Dermatol Venereol 2013;27:11-8. [PubMed]
- Søndergaard JN, Nazarian R, Wang Q, et al. Differential sensitivity of melanoma cell lines with BRAFV600E mutation to the specific Raf inhibitor PLX4032. J Transl Med 2010;8:39. [PubMed]
- Spagnolo F, Ghiorzo P, Queirolo P. Overcoming resistance to BRAF inhibition in BRAF-mutated metastatic melanoma. Oncotarget 2014;5:10206-21. [PubMed]
- Solit DB, Garraway LA, Pratilas CA, et al. BRAF mutation predicts sensitivity to MEK inhibition. Nature 2006;439:358-62. [PubMed]
- Collisson EA, De A, Suzuki H, et al. Treatment of metastatic melanoma with an orally available inhibitor of the Ras-Raf-MAPK cascade. Cancer Res 2003;63:5669-73. [PubMed]
- Grimaldi AM, Simeone E, Ascierto PA. The role of MEK inhibitors in the treatment of metastatic melanoma. Curr Opin Oncol 2014;26:196-203. [PubMed]
- LoRusso PM, Krishnamurthi SS, Rinehart JJ, et al. Phase I pharmacokinetic and pharmacodynamic study of the oral MAPK/ERK kinase inhibitor PD-0325901 in patients with advanced cancers. Clin Cancer Res 2010;16:1924-37. [PubMed]
- Smalley KS, Flaherty KT. Integrating BRAF/MEK inhibitors into combination therapy for melanoma. Br J Cancer 2009;100:431-5. [PubMed]
- Adjei AA, Cohen RB, Franklin W, et al. Phase I pharmacokinetic and pharmacodynamic study of the oral, small-molecule mitogen-activated protein kinase kinase 1/2 inhibitor AZD6244 (ARRY-142886) in patients with advanced cancers. J Clin Oncol 2008;26:2139-46. [PubMed]
- Robert C, Dummer R, Gutzmer R, et al. Selumetinib plus dacarbazine versus placebo plus dacarbazine as first-line treatment for BRAF-mutant metastatic melanoma: a phase 2 double-blind randomised study. Lancet Oncol 2013;14:733-40. [PubMed]
- Kirkwood JM, Bastholt L, Robert C, et al. Phase II, open-label, randomized trial of the MEK1/2 inhibitor selumetinib as monotherapy versus temozolomide in patients with advanced melanoma. Clin Cancer Res 2012;18:555-67. [PubMed]
- Mandalà M, Voit C. Targeting BRAF in melanoma: biological and clinical challenges. Crit Rev Oncol Hematol 2013;87:239-55. [PubMed]
- Infante JR, Fecher LA, Falchook GS, et al. Safety, pharmacokinetic, pharmacodynamic, and efficacy data for the oral MEK inhibitor trametinib: a phase 1 dose-escalation trial. Lancet Oncol 2012;13:773-81. [PubMed]
- Falchook GS, Lewis KD, Infante JR, et al. Activity of the oral MEK inhibitor trametinib in patients with advanced melanoma: a phase 1 dose-escalation trial. Lancet Oncol 2012;13:782-9. [PubMed]
- Kim KB, Kefford R, Pavlick AC, et al. Phase II study of the MEK1/MEK2 inhibitor Trametinib in patients with metastatic BRAF-mutant cutaneous melanoma previously treated with or without a BRAF inhibitor. J Clin Oncol 2013;31:482-9. [PubMed]
- Rahman A. Vemurafenib and cobimetinib in BRAF-mutated melanoma. Lancet Oncol 2014;15:e535. [PubMed]
- Gibney GT, Weber JS. Expanding targeted therapy to NRAS-mutated melanoma. Lancet Oncol 2013;14:186-8. [PubMed]
- Ascierto PA, Schadendorf D, Berking C, et al. MEK162 for patients with advanced melanoma harbouring NRAS or Val600 BRAF mutations: a non-randomised, open-label phase 2 study. Lancet Oncol 2013;14:249-56. [PubMed]
- Livingstone E, Zimmer L, Vaubel J, et al. BRAF, MEK and KIT inhibitors for melanoma: adverse events and their management. Chin Clin Oncol 2014;3:29. [PubMed]
- Flaherty KT, Infante JR, Daud A, et al. Combined BRAF and MEK inhibition in melanoma with BRAF V600 mutations. N Engl J Med. 2012;367:1694-703. [PubMed]
- Long GV, Stroyakovskiy D, Gogas H, et al. Combined BRAF and MEK inhibition versus BRAF inhibition alone in melanoma. N Engl J Med 2014;371:1877-88. [PubMed]
- Robert C, Karaszewska B, Schachter J, et al. Improved overall survival in melanoma with combined dabrafenib and trametinib. N Engl J Med 2015;372:30-9. [PubMed]
- Ribas A, Gonzalez R, Pavlick A, et al. Combination of vemurafenib and cobimetinib in patients with advanced BRAF(V600)-mutated melanoma: a phase 1b study. Lancet Oncol 2014;15:954-65. [PubMed]
- Larkin J, Ascierto PA, Dréno B, et al. Combined vemurafenib and cobimetinib in BRAF-mutated melanoma. N Engl J Med 2014;371:1867-76. [PubMed]
- Young K, Minchom A, Larkin J. BRIM-1, -2 and -3 trials: improved survival with vemurafenib in metastatic melanoma patients with a BRAF(V600E) mutation. Future Oncol 2012;8:499-507. [PubMed]
- McArthur GA, Chapman PB, Robert C, et al. Safety and efficacy of vemurafenib in BRAF(V600E) and BRAF(V600K) mutation-positive melanoma (BRIM-3): extended follow-up of a phase 3, randomised, open-label study. Lancet Oncol 2014;15:323-32. [PubMed]
- Johnson DB, Flaherty KT, Weber JS, et al. Combined BRAF (Dabrafenib) and MEK inhibition (Trametinib) in patients with BRAFV600-mutant melanoma experiencing progression with single-agent BRAF inhibitor. J Clin Oncol 2014;32:3697-704. [PubMed]
- Azer MW, Menzies AM, Haydu LE, et al. Patterns of response and progression in patients with BRAF-mutant melanoma metastatic to the brain who were treated with dabrafenib. Cancer 2014;120:530-6. [PubMed]
- Dzienis MR, Atkinson VG. Response rate to vemurafenib in patients with B-RAF-positive melanoma brain metastases: a retrospective review. Melanoma Res 2014;24:349-53. [PubMed]
- Merten R, Hecht M, Haderlein M, et al. Increased skin and mucosal toxicity in the combination of vemurafenib with radiation therapy. Strahlenther Onkol 2014;190:1169-72. [PubMed]