Advances in the diagnosis, treatment and prognosis of malignant pleural mesothelioma
Malignant pleural mesothelioma (MPM) is marked by its difficulty of diagnosis in the early stage, lacking effective treatment and poor prognosis. The incidence of MPM in China is approximately 0.1-0.6/10 million, lower than the global incidence, which might be caused by high rate of the acatalepsia and misdiagnosis of MPM. The incidence of MPM has a growing tendency worldwide (1), and the incidence is likely to peak between 2015 and 2030 (2). MPM occurs in any part of the parietal pleura and the visceral pleura, while about 80% occurs in the visceral pleura and 20% occurs in the parietal pleura (3). MPM affects predominately the elderly, who are older than 65 (4); however the disease may occur at any age. The most common etiology is asbestos exposure (5). As China has become the largest asbestos-consuming country in the world, it could give rise to a surge of MPM in the future. The production and the use of asbestos have been banned in most of the European and American countries (6). In addition, simian virus 40 (SV40), erionite, radiation and some chemicals may be important factors as well leading to MPM. The clinical manifestations of MPM are diverse. Rapid progress and swift deterioration in a few months are common clinical features of MPM. At present more effective treatment options for patients with MPM are under study, which are still to be overcome (7). Now the purpose of this paper is to have a review of MPM.
The pathogenesis
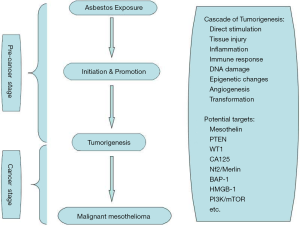
MPM was first reported by Miller in 1908, and the association between MPM and the exposure to asbestos was first proposed by Wagner in 1960. Statistics showed that 75-87% of the MPM patients have a history of asbestos exposure, mostly with crocidolite (8). The mechanism of the disease is still not entirely clear. Some scholars argue that the size and shape of the asbestos are more important than the role of the chemical traits in the carcinogenic mechanism, usually the asbestos fibers with small diameters are strong carcinogens. As the asbestos being inhaled into the lungs, it can form oxided iron bodies, which can be recognized but not be swallowed by the macrophages, causing a reactive hyperplasia of multinucleated marcrophages. The uncontrolled proliferation of multinucleated macrophages results in the mutation of mesothelial cells and ultimately, the development of cancer cells (9). As specific lymphocytes, natural killer (NK) cells have a role in identifying and killing tumor cells, but the activity could be inhibited by asbestos, which may be dose-dependent inhibition (10). This is also consistent with the experimental data that the MPM occurs in areas with a higher quantity of asbestos deposited. The results also confirm that asbestos was one of the causes leading to MPM (11). Puntoni et al. summarized 13 papers on malignant mesothelioma familial aggregation published from 1980 to 2003 (12). They found that only a few numbers of the family suffered from malignant mesothelioma (mostly arising from the pleura) despite common environments such as a history of asbestos exposure. Genetic factors should be taken into account while statistical analysis is performed. The latency period of MPM induced by asbestos is shorter than the general population, and the earlier the onset of disease, the shorter survival time. Non-asbestos-related MPM also has been reported in the world (13). In addition, long-term exposure to SV40, tuberculosis infection, genetic factors, contacting with the fiberglass, zeolite, radiation and other factors can also lead to the occurrence of MPM (14). Some of the molecular mechanisms of etiology of MPM are listed in Figure 1 (15,16).
The pathological morphology
There are three different pathological types of MPM: the epithelioid, the sarcomatoid and the biphasic type. The prognosis of the epithelial type is better than the non-epithelioid, and the sarcomatoid type has the worst prognosis (17). The epithelioid MPM showed a proliferation of oval or polygonal tumor cells, often lacking of nuclear fission, being lined by vascular structures formed by cuboial cells and the papillary structures with microvascular axis. The sarcomatoid type is mainly characterized by the proliferation of spindle cells with oval nuclear formation, a small amount of double-staining cytoplasm and the prominent nucleus. Some cases have fibrosarcoma-like morphology. The mixed type is a combination of the both characteristics as stated above. It also could be divided into the localized type and the diffuse type in terms of the growth pattern and the morphology of the tumor. The localized type have been proved that it originate from mesenchymal cells which have a fiber differentiation trend, instead of mesothelium (18). And it is generally believed that the localized type has nothing to do with smoking and exposure to asbestos (19).
Clinical manifestations
The clinical manifestations of MPM patients vary largely, typical symptoms including thoracodynia, dyspnea and pleural effusion, often accompanied with weight loss, weakness and other symptoms. The onset of symptoms are insidious and not specific, resulting in the high misdiagnosis rate. Relevant data have shown that most of the MPM patients have the primary symptom of phrenic nerve paralysis (20). The chest pain in MPM patients is a steady pain as a performance of disease progression. Mesothelioma often present with recurrent effusions, but one also has to recognize that not all mesotheliomas yield effusions. Pleural mesothelioma might last for years before diagnosis. In advanced stages, patients may develop symptoms from continued growth of MPM and suffer from tumor cachexia (21). Patients may also experience a leukemoid reaction, metabolic abnormalities and other symptoms caused by tumor metastasis (22).
Diagnosis
The diagnosis of MPM mainly relies on the pleural biopsy at present. The thoracoscopic pleural biopsy has become the most reliable method for the diagnosis and the antidiastole of MPM for its comprehensive observation, accurately obtaining the tissue specimens as well as the advantage of mini-invasive lesions. However Kao et al. (23) found that it is difficult to make a definite diagnosis through the pleural biopsy alone. And the accuracy of diagnosis of MPM could be improved when immunohistochemical examination is considered. The selection of immunohistochemical markers depends on the differentiated tumors, including sarcomatoid carcinoma and varieties of sarcomas. Cytokeratin (CK) has important significance in the diagnosis of mesothelioma, and a study has also shown that 92% of sarcomatoid mesothelioma is positive for CK (24). The related immunohistochemical markers of MPM also include calretinin (CR), D2-40, CK5/6, WT-1, VIM, CD105 and so on. The sensitivity of CR in the diagnosis of epithelioid mesothelioma is 94-100%, making it being a screening index of MPM. The positive expression of D20-40 may be used as one of the diagnostic references in sarcomatoid mesothelioma, but this also occurs in the lymphatic epithelial cells and reactive mesothelial cells, making the emergence of false positive, which also let us take a comprehensive consideration of the information at diagnosis (25). As one of the high specific index of MPM, CK5/6 is located on the plasmalemma, and mostly expressed in epithelioid mesothelioma. Vimentin (VIM) is mainly expressed in stromal cells and tumor-derived cells; it also can be expressed in MPM, especially in the sarcomatoid mesothelioma and the poorly mixed mesothelioma, which can be used for differential diagnosis with metastatic adenocarcinoma of lung. Similar to CR, VIM has better sensitivity and negative predictive value, which can make VIM be an additional screening parameter of pleural mesothelioma. Being an ideal evaluation of the tumor angiogenesis index, microvessel density (MVD) takes CD105 as a specific marker, which has a dramatic increase in MPM patients, indicating that CD105 can be used as an auxiliary index for the diagnosis at the early stage (26). In addition, clinical image and other immunohistochemical markers are valuable to make exclusive diagnosis when the expression of CK is negative, even though CR, D2-40, CK5/6, WT-1, VIM, and CD105 were positive. However, MPM should be considered with priority when the tumor occurs in the pleura, regardless of the microscopic characteristics of the tumor.
The imaging diagnosis of MPM is based on X-ray, CT, PET/CT and MRI. X-ray is the most simple and practical method, which manifests pleural thickening, pleural nodules or pleural effusion. Because of the lack of specificity, the X-ray can’t make a confirmed diagnosis for MPM. Chest CT is commonly used in the preferred examination, which can display the surface of the whole pleural, the diaphragm and the status of lymph node (27). The specificity of chest CT is about 88-95%, while the sensitivity is about 36-45% (28). The chest CT mainly manifests a nodular or cyclic thickening of the pleura, the mediastinal pleural involvement with different degrees of pleural effusion and the enhanced CT images can be characterized by the multiple reinforcement nodules on the pleural. But it is still difficult in the tumor staging and distinguishing the diffuse pleural thickening from MPM (28). The imaging features and the sensitivity of MRI are similar to the chest CT, and MRI is mainly used for the preoperative assessment of mediastinal structures, chest wall and diaphragm involvement, which is not used as a routine examination. PET/CT can be used for the diagnosis of MPM as well using 18F-fluorodeoxyglucose (18FDG). The characteristic images of MPM is the concentration of 18FDG at different levels in lesions, which can make up the deficiency of CT images in MPM staging and play an important role in the diagnosis, curative evaluation, monitoring and estimating prognosis (29). Studies from Rohren et al. have shown that the sensitivity of PET/CT is about 88.2%, and the specificity is about 92.9% (30). Despite the unique features of PET/CT, there are still some deficiencies such as the high expense and the false positive (31). The diagnosis still requires a combination of patients with clinical manifestations and other experimental results. Thoracoscopy as a real-time imaging technique enables clinicians to improve tumor staging, particularly in the mediastinal region, by enabling the exact sampling point with adequate tissue for biopsy. Thoracoscopy also can be used for pleurectomy and pleurodesis. Considering all the advantages above, thoracoscopy is considered the best way for making diagnosis of MPM (32).
Treatment
MPM is characterized by highly malignant and aggressive. If left untreated, the median survival time of the patients is about 4 to 12 months (33). Most patients died of the local invasion of the tumor or the contralateral metastasis. A variety of treatments have been taken by domestic and foreign scholars, and the comprehensive treatment of resecting the visible tumor as much as possible, combined with radiotherapy, chemotherapy and immunotherapy is the most promising strategy up to date (34,35). Unfortunately, the efficacy of treatment is very limited for the tumor is usually diagnosed too late. All of the current therapies are only expected to improve the quality of life and prolong survival time of the patients with MPM (36).
Surgical treatment
The purpose of surgical treatment is to remove the visible tumor, to eliminate the pleural effusion, to relieve symptoms such as dyspnea, to ease the pain, and to increase the efficacy of adjuvant therapy (37). Extrapleural pneumonectomy (EPP) and pleurectomy/decortication (P/D) are the most commonly used operation modes so far (38). EPP requires complete resection of the affected visceral pleura and parietal pleura, the lungs, the diaphragm, and even part of the pericardium. The trauma caused by EPP is quite large, and the perioperative mortality is relatively high, which used to be as high as 32%. With the development of the surgical techniques and further screening of the indications, the mortality in perioperative period has decreased to 4%. P/D requires the complete resection of the visceral and parietal pleura, and the retention of the lung. The trauma caused by P/D is relatively small, and the perioperative mortality rate is about 1.5-5.4%. There are limitations on the tumor deactivation of P/D, especially when the tumor invaded interlobar fissure and other parts (39). Compared with P/D, EPP does not prolong the survival instead of increasing the postoperative complications for the MPM patients at the early stage (40). Above all, surgery is not recommended if progressive disease is observed after neoadjuvant chemotherapy; besides, EEP is only recommended in the context of controlled clinical trials performed by specialized teams of investigators (41).
Radiotherapy
Mesothelioma is not sensitive to radiotherapy and the radiotherapy effect is generally unsatisfactory. Also, it is extremely difficult to draw up the effective radiation dose and the scope of the radiation, due to the unique way that MPM spreads along the pleura, surrounding the lungs, adjacent to the heart, the spinal, the esophagus and other vital organs, which might be one of the reasons for its unsatisfied therapeutic effect (42). Nowadays, with the development of three-dimensional conformal techniques, and the increasing popularity of radiotherapy and intensity-modulated radiation therapy (IMRT), this problem has been solved to some extent (42). Radiotherapy has a certain effects in relieving the symptoms, especially easing the pain (43). There is still controversy that prophylactic irradiation which aims at the puncture point or the biopsy site could reduce the transfer rate (44-46). There is still no sufficient evidence-based medicine about the radiation of MPM that large-scale clinical researches are still needed to be carried out.
Chemotherapy
The purpose of chemotherapy is to kill the rapid-proliferating tumor cells, so as to prolong the survival and improve the quality of life (17). Chemotherapy for MPM can be used alone or combined with surgical treatment. Symptoms of MPM patients usually appear at an advanced stage, and chemotherapy has become the preferred solution. The US Food and Drug Administration (FDA) and the European Union (EU) approved pemetrexed (PEM) being used in the treatment of MPM in 2004, and the combination of cisplatin (CDDP) and PEM become the first-line chemotherapeutic drugs for MPM (47). A magazine published a research results about the phase III clinical trial of chemotherapy in MPM patients in 2003: the median survival time was about 9.3 months when CDDP is used alone, but it could be increased to 12.1 months when the therapeutic regimen include CDDP and PEM, the response rates also could be increased from 16.7% to 41.3% (47,48). Treatment with vitamin B12 and folic acid could also reduce toxicity without adversely affecting survival time. PEM and carboplatin plus bevacizumab as the first-line chemotherapeutic regimen of MPM has been raised by some experts, but validating reports about the role of bevacizumab is needed (49). In addition to systemic chemotherapy, intrathoracic chemotherapy is usually the main treatment for pleural effusion.
Immunotherapy
Based on preclinical studies and clinical trials, the role of immunotherapy in cancer treatment has been aware increasingly reported. Tannock et al. had found that cancer cell repopulation during the intervals of radiotherapy or chemotherapy was associated with treatment failure (50). A potential approach is to eliminate the surviving cancer cells by the antitumor immunity. Tumor cells can stimulate the immune system generating immune killer cells, such as the effector T cells (Treg), and it could also induce immunosuppressive cells and immunosuppressive factors, which can adjust the generating of regulatory Treg, myeloid derived suppressor cells (MDSCs), programmed death-1 (PD1) and cytotoxic T lymphocyte-associated antigen-4 (CTLA-4) (51). Treg is induced by the subpopulation of CD4 and CD25 in thymus, and it could be evoked in the periphery and serve to maintain the homeostasis and the silence of immune. Treg cells account for 2-4% of the total peripheral Treg in human, which can secrete immunosuppressive factors and block the function of effector Treg and antigen presentation (52). Studies carried out by Wu et al. have shown that there is a high infiltration of Treg in mesothelioma patients, playing a role in promoting the cancer by inhibiting the function of Treg and the secretion of immunosuppressive cytokines such as interleukin-10 (IL-10) and transforming growth factor-β (TGF-β) (53). The number of Treg in the tissue and peripheral blood of patients with MPM are increased, which can promote the further spread of MPM. MDSCs can inhibit the immune response mediated by Treg, which derive from the bone narrow cells and accumulate in the body of the patients with MPM. PD-1 and programmed death ligand (PD-L1/2) are negative regulatory signals of the immune response, belonging to the family of B7 costimulatory molecules, and the blockade of the inhibitory signals would be able to induce the body’s anti-tumor immunity against cancer (54). Nowadays, the anticancer drugs targeted PD-L1/2 are undergoing clinical trials (55). CTLA-4, also known as CD152, is a leukocyte differentiation antigen involved in negative regulation of the immune response. Wu et al. demonstrated that the blockade of CTLA-4 signaling had effective anticancer effect, which also brought us a new hope in the treatment of MPM through modulation of T cell immunity (56). DC-based immunotherapy has shown promising in a wide variety of cancers including mesothelioma (57). Lack of specific antigen made it difficult to develop antigen-specific immunotherapy on mesothelioma; however, it would shed a light of targeting tumor cells through intratumoral DC vaccination.
Molecular targeted therapy
Significant progress has been made in molecular targeted therapy in non-small cell lung cancer, and this might be the same in MPM (58). Most of the researches are focused on the epidermal growth factor receptor (EGFR) and vascular endothelial growth factor (VEGF). The main role of EGFR tyrosine kinase inhibitors is to inhibit the cell proliferation and phosphorylation, which plays a critical role in anti-tumor activity (59). A phase II trial of gemcitabine/cisplatin plus the anti-VEGF antibody bevacizumab in patients with previously untreated, unresectable MPM carried out by Kindler et al. (60) have shown that the addition of bevacizumab to gemcitabine/cisplatin in this trial did not significantly improve PFS or OS in patients with advanced. Also, Garland et al. (61) published a phase II trial about the EGFR TKI erlotinib in previously untreated patients with MPM. The paper showed that single-agent erlotinib was not effective in MPM, despite high expression of EGFR. Despite all this, with the further exploration in the molecular biology mechanism of MPM, molecular targeted therapy will play an important role in the treatment of MPM in the future.
Concluding remark
The incidence of MPM is increasing in the recent years; however, it is difficult to make diagnosis at the early stage. The efficacy of current therapies is very limited unfortunately, thus the prognosis is quite poor. In order to improve the efficacy of MPM patients, early diagnosis and effective treatment strategies will be highly expected to develop. Unlike other cancers, MPM has not been well characterized in many aspects. Therefore, it is extremely important to develop novel approaches to the diagnosis, treatment and prognosis for the disease. We are optimistic to the combination of conventional therapy with immunotherapy, such as modulation of T cell immunity against tumor through targeting the immune checkpoints (CTLA-4, PD-1/PD-L1/2), depletion of the immune suppressive components (Treg, MDSC), and DC-based immunotherapy. In addition, anti-angiogenic therapy might be promising as well in the control of tumor development and progression. Current studies have shed a light of hope to the improvement of MPM treatment. We are looking forward to more clinical trials that would generate satisfying benefit to the disease.
Acknowledgements
We would like to thank Ms. Katrina Rey-McIntyre for reviewing this paper and making careful modification of the language.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Cihan YB, Ozturk A, Arslan A, et al. ERCC1 as a biological marker guiding management in malignant pleural mesothelioma. Asian Pac J Cancer Prev 2014;15:4117-23. [PubMed]
- Neumann V, Löseke S, Nowak D, et al. Malignant pleural mesothelioma: incidence, etiology, diagnosis, treatment, and occupational health. Dtsch Arztebl Int 2013;110:319-26. [PubMed]
- Mery É, Hommell-Fontaine J, Capovilla M, et al. Peritoneal malignant mesothelioma: review and recent data. Ann Pathol 2014;34:26-33. [PubMed]
- Price B. Analysis of current trends in United States mesothelioma incidence. Am J Epidemiol 1997;145:211-8. [PubMed]
- Acton V. Preventing pleural mesothelioma in patients with recognizable asbestos-related pleural plaques. J Thorac Cardiovasc Surg 2014;148:1763. [PubMed]
- Lin RT, Takahashi K, Karjalainen A, et al. Ecological association between asbestos-related diseases and historical asbestos consumption: an international analysis. Lancet 2007;369:844-9. [PubMed]
- Yoshino I, Yamaguchi M, Okamoto T, et al. Multimodal treatment for resectable epithelial type malignant pleural mesothelioma. World J Surg Oncol. 2004;2:11. [PubMed]
- Linton A, Pavlakis N, O'Connell R, et al. Factors associated with survival in a large series of patients with malignant pleural mesothelioma in New South Wales. Br J Cancer 2014;111:1860-9. [PubMed]
- Carbone M, Ly BH, Dodson RF, et al. Malignant mesothelioma: facts, myths, and hypotheses. J Cell Physiol 2012;227:44-58. [PubMed]
- Altomare E, Fallarini S, Biaggi G, et al. Increased frequency of circulating invariant natural killer T cells in malignant pleural mesothelioma patients. Cancer Biol Ther 2012;13:702-11. [PubMed]
- Røe OD, Stella GM. Malignant pleural mesothelioma: history, controversy and future of a manmade epidemic. Eur Respir Rev 2015;24:115-31. [PubMed]
- Puntoni R, Filiberti R, Cerrano PG, et al. Implementation of a molecular epidemiology approach to human pleural malignant mesothelioma. Mutat Res 2003;544:385-96. [PubMed]
- Kanbay A, Ozer Simsek Z, Tutar N, et al. Non-asbestos-related malignant pleural mesothelioma. Intern Med 2014;53:1977-9. [PubMed]
- García-Gómez M, Menéndez-Navarro A, López RC. Asbestos-related occupational cancers compensated under the Spanish National Insurance System, 1978-2011. Int J Occup Environ Health 2015;21:31-9. [PubMed]
- Battaglia A. The Importance of Multidisciplinary Approach in Early Detection of BAP1 Tumor Predisposition Syndrome: Clinical Management and Risk Assessment. Clin Med Insights Oncol 2014;8:37-47. [PubMed]
- Zhou S, Liu L, Li H, et al. Multipoint targeting of the PI3K/mTOR pathway in mesothelioma. Br J Cancer 2014;110:2479-88. [PubMed]
- Van Schil PE, Opitz I, Weder W, et al. Multimodal management of malignant pleural mesothelioma: where are we today? Eur Respir J 2014;44:754-64. [PubMed]
- Hanau CA, Miettinen M. Solitary fibrous tumor: histological and immunohistochemical spectrum of benign and malignant variants presenting at different sites. Hum Pathol 1995;26:440-9. [PubMed]
- England DM, Hochholzer L, McCarthy MJ. Localized benign and malignant fibrous tumors of the pleura. A clinicopathologic review of 223 cases. Am J Surg Pathol 1989;13:640-58. [PubMed]
- Szulkin A, Otvös R, Hillerdal CO, et al. Characterization and drug sensitivity profiling of primary malignant mesothelioma cells from pleural effusions. BMC Cancer 2014;14:709. [PubMed]
- Jaklitsch MT, Grondin SC, Sugarbaker DJ. Treatment of malignant mesothelioma. World J Surg 2001;25:210-7. [PubMed]
- Adams VI, Unni KK, Muhm JR, et al. Diffuse malignant mesothelioma of pleura. Diagnosis and survival in 92 cases. Cancer 1986;58:1540-51. [PubMed]
- Kao SC, Klebe S, Henderson DW, et al. Low calretinin expression and high neutrophil-to-lymphocyte ratio are poor prognostic factors in patients with malignant mesothelioma undergoing extrapleural pneumonectomy. J Thorac Oncol 2011;6:1923-9. [PubMed]
- Klebe S, Brownlee NA, Mahar A, et al. Sarcomatoid mesothelioma: a clinical-pathologic correlation of 326 cases. Mod Pathol 2010;23:470-9. [PubMed]
- Takeshima Y, Amatya VJ, Kushitani K, et al. Value of immunohistochemistry in the differential diagnosis of pleural sarcomatoid mesothelioma from lung sarcomatoid carcinoma. Histopathology 2009;54:667-76. [PubMed]
- Lovato A, Marioni G, Manzato E, et al. Elderly patients at higher risk of laryngeal carcinoma recurrence could be identified by a panel of two biomarkers (nm23-H1 and CD105) and pN+ status. Eur Arch Otorhinolaryngol 2014. [PubMed]
- Salahudeen HM, Hoey ET, Robertson RJ, et al. CT appearances of pleural tumours. Clin Radiol 2009;64:918-30. [PubMed]
- Tateishi U, Gladish GW, Kusumoto M, et al. Chest wall tumors: radiologic findings and pathologic correlation: part 2. Malignant tumors. Radiographics 2003;23:1491-508. [PubMed]
- Fuccio C, Spinapolice EG, Ferretti A, et al. 18F-FDG-PET/CT in malignant mesothelioma. Biomed Pharmacother 2013;67:539-42. [PubMed]
- Rohren EM, Turkington TG, Coleman RE. Clinical applications of PET in oncology. Radiology 2004;231:305-32. [PubMed]
- Nakamori T, Kosuda S, Kyoto Y, et al. Pseudomesotheliomatous lung cancer mimicking mesothelioma on 18F-FDG PET/CT images: report of 2 cases. Jpn J Radiol 2013;31:542-5. [PubMed]
- Rodríguez Panadero F. Diagnosis and treatment of malignant pleural mesothelioma. Arch Bronconeumol 2015;51:177-84. [PubMed]
- Jaklitsch MT, Grondin SC, Sugarbaker DJ. Treatment of malignant mesothelioma. World J Surg 2001;25:210-7. [PubMed]
- Rusch VW. A phase II study of intrapleural immunochemotherapy, pleurectomy/decortication, radiotherapy, systemic chemotherapy, and long-term subcutaneous IL-2 in stage II-III malignant pleural mesothelioma. Eur J Cardiothorac Surg 2007;31:534-5. [PubMed]
- Krug LM, Pass HI, Rusch VW, et al. Multicenter phase II trial of neoadjuvant pemetrexed plus cisplatin followed by extrapleural pneumonectomy and radiation for malignant pleural mesothelioma. J Clin Oncol 2009;27:3007-13. [PubMed]
- Rosenzweig KE. Current readings: improvements in intensity-modulated radiation therapy for malignant pleural mesothelioma. Semin Thorac Cardiovasc Surg 2013;25:245-50. [PubMed]
- Flores RM. Surgical options in malignant pleural mesothelioma: extrapleural pneumonectomy or pleurectomy/decortication. Semin Thorac Cardiovasc Surg 2009;21:149-53. [PubMed]
- Kotova S, Wong RM, Cameron RB. New and emerging therapeutic options for malignant pleural mesothelioma: review of early clinical trials. Cancer Manag Res 2015;7:51-63. [PubMed]
- Papaspyros S, Papaspyros S. Surgical management of malignant pleural mesothelioma: impact of surgery on survival and quality of life-relation to chemotherapy, radiotherapy, and alternative therapies. ISRN Surg 2014;2014:817203.
- Rena O, Casadio C. Extrapleural pneumonectomy for early stage malignant pleural mesothelioma: a harmful procedure. Lung Cancer 2012;77:151-5. [PubMed]
- van Zandwijk N, Clarke C, Henderson D, et al. Guidelines for the diagnosis and treatment of malignant pleural mesothelioma. J Thorac Dis 2013;5:E254-307. [PubMed]
- Opitz I. Management of malignant pleural mesothelioma-The European experience. J Thorac Dis 2014;6 Suppl 2:S238-52. [PubMed]
- Baldini EH. Radiation therapy options for malignant pleural mesothelioma. Semin Thorac Cardiovasc Surg 2009;21:159-63. [PubMed]
- Tomek S, Emri S, Krejcy K, et al. Chemotherapy for malignant pleural mesothelioma: past results and recent developments. Br J Cancer 2003;88:167-74. [PubMed]
- O'Rourke N, Garcia JC, Paul J, et al. A randomised controlled trial of intervention site radiotherapy in malignant pleural mesothelioma. Radiother Oncol 2007;84:18-22. [PubMed]
- Boutin C, Rey F, Viallat JR. Prevention of malignant seeding after invasive diagnostic procedures in patients with pleural mesothelioma. A randomized trial of local radiotherapy. Chest 1995;108:754-8. [PubMed]
- Katirtzoglou N, Gkiozos I, Makrilia N, et al. Carboplatin plus pemetrexed as first-line treatment of patients with malignant pleural mesothelioma: a phase II study. Clin Lung Cancer 2010;11:30-5. [PubMed]
- Vogelzang NJ, Rusthoven JJ, Symanowski J, et al. Phase III study of pemetrexed in combination with cisplatin versus cisplatin alone in patients with malignant pleural mesothelioma. J Clin Oncol 2003;21:2636-44. [PubMed]
- Ceresoli GL, Zucali PA, Mencoboni M, et al. Phase II study of pemetrexed and carboplatin plus bevacizumab as first-line therapy in malignant pleural mesothelioma. Br J Cancer 2013;109:552-8. [PubMed]
- Tannock IF, Lee CM, Tunggal JK, et al. Limited penetration of anticancer drugs through tumor tissue: a potential cause of resistance of solid tumors to chemotherapy. Clin Cancer Res 2002;8:878-84. [PubMed]
- Anagnostou VK, Brahmer JR. Cancer immunotherapy: a future paradigm shift in the treatment of non-small cell lung cancer. Clin Cancer Res 2015;21:976-84. [PubMed]
- Anraku M, Tagawa T, Wu L, et al. Synergistic antitumor effects of regulatory T cell blockade combined with pemetrexed in murine malignant mesothelioma. J Immunol 2010;185:956-66. [PubMed]
- Wu L, Yun Z, Tagawa T, et al. Tumor cell repopulation between cycles of chemotherapy is inhibited by regulatory T-cell depletion in a murine mesothelioma model. J Thorac Oncol 2011;6:1578-86. [PubMed]
- Xu P, Chen H, Chen YJ, et al. Expression of PD-1/PD-L1 in peripheral blood mononuclear cells in lung cancer patients and its biological significance. Zhonghua Zhong Liu Za Zhi 2013;35:910-3. [PubMed]
- Wei T, Zhang J, Wu Y, et al. Expression levels of co-inhibitory molecules CTLA-4, LAG-3, PD-1 and CD39 on CD4+ T cells correlate with progression of non-small cell lung cancer. Zhonghua Zhong Liu Za Zhi 2014;36:424-9. [PubMed]
- Wu L, Yun Z, Tagawa T, et al. CTLA-4 blockade expands infiltrating T cells and inhibits cancer cell repopulation during the intervals of chemotherapy in murine mesothelioma. Mol Cancer Ther 2012;11:1809-19. [PubMed]
- Cornelissen R, Lievense LA, Heuvers ME, et al. Dendritic cell-based immunotherapy in mesothelioma. Immunotherapy 2012;4:1011-22. [PubMed]
- Kumarakulasinghe NB, van Zanwijk N, Soo RA. Molecular targeted therapy in the treatment of advanced stage non-small cell lung cancer (NSCLC). Respirology 2015;20:370-8. [PubMed]
- Matikas A, Mistriotis D, Georgoulias V, et al. Current and Future Approaches in the Management of Non-Small-Cell Lung Cancer Patients With Resistance to EGFR TKIs. Clin Lung Cancer 2015;16:252-61. [PubMed]
- Kindler HL, Karrison TG, Gandara DR, et al. Multicenter, double-blind, placebo-controlled, randomized phase II trial of gemcitabine/cisplatin plus bevacizumab or placebo in patients with malignant mesothelioma. J Clin Oncol 2012;30:2509-15. [PubMed]
- Garland LL, Rankin C, Gandara DR, et al. Phase II study of erlotinib in patients with malignant pleural mesothelioma: a Southwest Oncology Group Study. J Clin Oncol 2007;25:2406-13. [PubMed]