Expert consensus statement on parathyroid protection in thyroidectomy
Introduction
Thyroid cancer is the solid cancer with the most rapidly increasing incidence rate around the world. In 2012, the incidence rate of this condition has been the highest among malignancies in Korean women (1), ranking fourth in China (2). The most common pathological type is papillary thyroid carcinoma, accounting for about 80-85% of all thyroid cancers. Lymph node metastases are common in early papillary thyroid carcinoma, with a reported cervical metastasis rate of about 21-90% (3-5). Most investigators believe that the central zone is the first site of lymph node metastases, namely the sentinel lymph node (6,7). At present, surgery is the preferred treatment for thyroid cancer. Among others, total thyroidectomy with central lymph node dissection has become the most common surgical approach. The main complications are recurrent laryngeal nerve and parathyroid injury. The clinical application of neural monitors has played a positive role in intraoperative positioning and protection of the recurrent laryngeal nerve. Postoperative hypoparathyroidism due to parathyroid injury is still a challenge to thyroid surgeons. Parathyroid injuries include bruising, insufficient blood supply and mistaken incision. It is reported that the incidence rates of temporary and permanent hypoparathyroidism are 14-60% and 4-11% after thyroidectomy, respectively (8-15). A study showed that the incidence rates of transient and permanent hypoparathyroidism were 27.7% and 6.3% after total thyroidectomy, 36.1% and 7.0% after total resection with unilateral central lymph node dissection, and 51.9% and 16.2% after total thyroidectomy with bilateral central lymph node dissection, respectively (16). Temporary hypoparathyroidism may cause transient hypocalcemia symptoms, but will not have a large impact on the quality of life of patients. On the other hand, permanent hypoparathyroidism will cause permanent hypocalcemia symptoms, mostly limb numbness and spasms, seriously affecting the quality of life of patients, which is a main factor of medical disputes. Therefore, we should pay attention to the protection of the parathyroid gland during thyroid surgery.
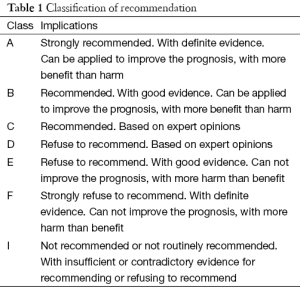
To improve the safety and efficacy of thyroid surgery, and further reduce the incidence of postoperative hypoparathyroidism, the Chinese Thyroid Association of the Chinese Medical Association organized some experts to develop this Expert Consensus on Parathyroid Protection in Thyroidectomy. The consensus applies to all open and endoscopic thyroid surgery. The classification of recommendations is listed in Table 1.
Full table
Applied anatomy, physiological functions and types of the parathyroid
Applied anatomy
The parathyroid consists of endocrine gland. Most of them are flat oval bodies and in brown when alive. Then are similar to rice or flattened soybeans in a diameter of about 3-6 mm, which are wrapped in a thin layer of connective tissue. The number of parathyroid glands is indefinite. It is reported that 48-62% of the Chinese people have four of these glands, but there may be variation resulting in more or fewer than four, and investigators have even reported that about 15% just have two (17,18). Most parathyroid glands are tightly attached to the surface of the left and right thyroid lobes, often located in the fiber capsule between the natural and surgical coatings. The location of the upper parathyroid gland is relatively constant, 85% concentrated in the circle with a radius of 1 cm area with the lower corner of the thyroid cartilage as the center. The position of the lower parathyroid glands vary more often, with more than half of them located at the lower 1/3 of the junction of the posterior edge of the thyroid and the rest at the anterior of the thyroid, or inside the thymus, the mediastinum or the thyroid parenchyma. Most parathyroid glands have independent parathyroid arteries. There are usually three sources of blood supply to the upper parathyroid glands: (I) posterior branches of the upper thyroid artery, as the main blood supply source; (II) anastomotic branches of the upper and lower thyroid arteries; and (III) the lowest thyroid artery and the arteries at the larynx, trachea, esophagus, and so on. The major blood supply to the lower parathyroid glands derives mainly from the lower thyroid artery. The blood supply to the upper and lower parathyroid glands starts from the upper and lower thyroid arteries before they enter the thyroid tissue. Therefore, to ensure the parathyroid blood supply, the third-tier terminal blood vessels should be treated close to the natural thyroid capsule (true capsule), rather than ligating the trunk of the upper and lower thyroid arteries.
Recommendation 1: to effectively reserve parathyroid blood supply, the upper and lower thyroid arteries should be treated close to the natural thyroid capsule properly (class A).
Physiological function
Parathyroid glands secrete parathyroid hormone (PTH), whose main target organs are the bone and the kidney, with indirect intestinal effects as well.
The physiological function of PTH is to regulate calcium metabolism and maintain the balance of calcium and phosphorus. By acting on osteoclasts, it causes bone calcium to dissolve and release into the blood circulation, thus elevating blood calcium and phosphorus concentrations. When the calcium, phosphorus concentrations exceed the renal threshold, they will be excreted in the urine, leading to hypercalciuria and hyperphosphaturia. PTH can also inhibit renal tubular reabsorption of phosphorus, resulting in increased urinary phosphorus and decreased blood phosphorus. Therefore, in the case of postoperative hypoparathyroidism, hypocalcemia with hyperphosphatemia can occur, leading to numbness and even convulsions. Under normal circumstances, retaining one or more parathyroid glands with good blood supply will almost prevent serious permanent hypoparathyroidism after surgery (19). Thus, the overall policy for parathyroid protection in thyroidectomy should follow the “1+ X” principle. “1” means that we should treat every single parathyroid gland as the only (last) one, and treat and protect it carefully in the dissection; it also means that we have to identify at least one parathyroid gland in each thyroid surgery; “X” means that we are supposed to strive to protect more parathyroid glands during the surgery. That’s because we have no idea how many parathyroid glands a patient may have, and even no idea as to which one is playing the major role. Meanwhile, we may encounter a patient who has only two parathyroid glands, and they may be located on the same side. Therefore, even if only one side is involved in the thyroid surgery, we should still pay attention to parathyroid protection.
Recommendation 2: parathyroid protection in thyroidectomy should follow the “1+ X” principle (class C).
Recommendation 3: at least one parathyroid gland with good blood supply should be reserved in situ as much as possible in each case of thyroid surgery (class A).
Types
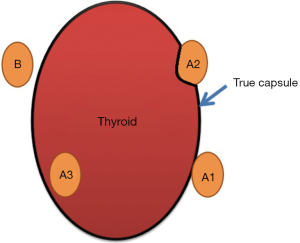
Zhu et al. classified the parathyroid glands to two types, A and B, based on the positional relationship between the thyroid and the parathyroid, and the difficulty to reserve a gland in situ (Figure 1). Type A is the compact type, meaning that the thyroid and parathyroid glands are closely situated, making it relatively difficult to retain them in situ. This is further divided into three subtypes. Type A1: the parathyroid glands are attached to the surface of the thyroid (Figure 2A); type A2: the parathyroid glands are partially or fully embedded in the thyroid, but located outside the natural capsule (Figure 2B); type A3: the parathyroid glands are entirely within the thyroid tissue (Figure 2C) and inside the natural capsule, which is different from type A2. Type B is the non-compact type, meaning that there is a natural gap between the parathyroid and the thyroid, making it easier to retain in situ. It is also divided into three subtypes. Type B1: peripheral thyroid type (Figure 2D), including all type B parathyroid glands except B2 and B3; type B2: intra-thymus type (Figure 2E), in which the parathyroid glands are located in the thymus; type B3: blood supply from vessels of the thymus or mediastinum (Figure 2F). Therefore, in theory, it is easier to retain type B than type A, and type A1 than type A2, in situ. It is not possible to reserve type A3 in situ.
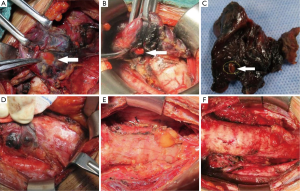
Recommended 4: typing of the parathyroid glands facilitates statistics and communication, and is also conducive to determine the degree of difficulty to reserve the parathyroid glands in situ. Therefore, in theory, it is easier to retain type B than type A, and type A1 than type A2, in situ. It is not possible to reserve type A3 in situ (class C).
Visual identification of parathyroid glands and tips
Visually identification
The parathyroid glands can be identified by the naked eye, positive development and negative development and other methods, but the most important thing is to learn how to visually identify them. Comprehensive judgments should be made based on the parathyroid anatomy, appearance and tolerance to ischemia, and so on. The parathyroid glands are usually not readily distinguishable from fat droplets, lymph nodes, ectopic thymus and thyroid. (I) Distinguishing between parathyroid glands and fat droplets. Since the majority of parathyroid glands are partially or completely wrapped in outer peripheral fat tissue, it is difficult to distinguish between the two. The defining aspects are: (i) color, parathyroid glands are usually yellowish brown or brown (depending on the amount of primary cells), while fat droplets are pale yellow; (ii) capsule, the fat tissue surrounding parathyroid glands has a complete capsule, which can be opened to expose yellowish brown or brown parathyroid glands with a scalpel. In contrast, fat droplets do not have such structure and do not contain brown tissue when dissected. (II) Distinguishing between parathyroid glands and lymph nodes. The defining aspects are: (i) color, parathyroid glands are brown or tan, while lymph nodes are pink (flesh) and some pale; (ii) thickness, this is one of key points in distinguishing between parathyroid glands and lymph nodes. In general, the thickness of the parathyroid is smaller compared with the length and width, usually at only 1-2 mm and rarely >3 mm, whereas lymph nodes are thicker with similar length and width; (iii) texture, the parathyroid is soft while lymph nodes are relatively hard, especially those with lymph node metastasis, followed by those complicated with Hashimoto’s thyroiditis; (iv) appearance, parathyroid glands appear in good colors and moist, while lymph nodes appear to be poor and not moist; (v) surface, parathyroid glands have a more regular shape, smooth surface, with regular small structures, whereas lymph nodes may be irregular and less smooth with an uneven surface, which is more evident under a magnifying glass. (III) Distinguishing the parathyroid from scattered thymus and thyroid nodules. The defining aspects are: (i) color, parathyroid glands are brown or tan, while scattered thymus tissue is often crimson. The thyroid tissue is the same as the thyroid tissue in situ; (ii) shape, scattered thymus and thyroid nodules are often thick, with similar length and width; (iii) size, the maximum diameter of a normal parathyroid gland is generally <6 mm and rarely >8 mm, while scattered thymus and thyroid nodules are often about 10 mm. In addition, parathyroid glands are sensitive to blood supply changes. When the arteries are injured, they become lighter in color or even pale; when the veins are injured, they become purple due to venous congestion. On the other hand, lymph nodes, fat droplets and scattered thymus and thyroid tissue are not as sensitive to blood supply changes. Therefore, if a purple nodule is found during surgery which is not supposed to be present naturally, it should be highly suspected of a parathyroid gland with congestion. If it is not possible to distinguish between parathyroid tissue and the above, intraoperative frozen pathology should follow.
Recommendation 5: comprehensive judgments should be made based on visual inspection of the parathyroid anatomy, appearance (color, tone, appearance, size and thickness) and tolerance to ischemia (class C).
Parathyroid positive development
With parathyroid positive development, a developer is used for parathyroid staining to make it easy to accurately identify parathyroid glands in surgery. The mostly reported positive parathyroid developer is methylene blue. Since Dudley identified the parathyroid glands using intravenous injection with methylene blue in neck dissection in 1971, there have been a large number of clinical reports of parathyroid exposure using preoperative peripheral intravenous injection with methylene blue in surgery. However, more and more studies show that, while pathological parathyroid tissue is prone to methylene blue staining, normal parathyroid tissue is not sensitive to staining, making this method not effective in practice. At the same time, methylene blue is not an approved developer and lymphatic tracer, and is associated with some side effects, such as heart disorders, slow movement disorders, neurotoxicity and mental disorders. Thus, in recent years, there have been few reports of methylene blue staining for intraoperative identification of parathyroid glands, especially normal parathyroid glands.
Recommended 6: methylene blue is not suitable for the identification of normal parathyroid glands in thyroid surgery (class D).
Nanometer carbon negative development for parathyroid identification and protection
Mechanism and clinical application
Carbon nanoparticle suspension injection (referred to as nano-carbon) is a suspension made of nanoscale carbon particles, with a particle diameter of 150 nm and a high tropism for the lymphatic system (20). Because the clearance between capillary endothelial cells is 20-50 nm, and the gap between capillary lymphatic endothelial cells is 120-500 nm with a hypoplastic base, the carbon nanoparticles injected into the thyroid tissue will not enter the blood vessels, but can quickly enter the lymph tubes or lymphatic capillaries after being swollen by macrophages. As they gather and deposit in the lymph nodes, the thyroid and its lymph drainage area will be dyed black (21). Compared with methylene blue and other developers, carbon nanoparticles are associated with strong lymphatic tropism, high tracing speed, high black dye rate, long duration, and high contrast with the surrounding tissue color.
Since the most parathyroid glands are located in the central area and do not accept thyroid lymphatic drainage, after injection of nano-carbon in the thyroid tissue, most lymphatic vessels and lymph nodes in the thyroid and its drainage area will be stained in black, but parathyroid glands will not, making them easily distinguishable from the former. Black staining of the thyroid facilitates the recognition of types A1, A2 and A3 parathyroid glands, while that of the central lymph nodes facilitates the recognition of type B1 glands. Zhu et al. (22) called it the technology of “nanometer carbon negative development for parathyroid identification and protection”. So far, there have been tens of thousands of cases undergoing the nano-carbon technique and no adverse reaction has been reported. Zeng et al. (23) classified 80 patients of thyroid cancer randomly into the control group and the nano-carbon group to receive total thyroidectomy with central lymph node dissection or total thyroidectomy and modified neck dissection by the same team of surgeons, respectively. In the control group, 11 parathyroid glands were mistakenly removed, and 14 cases of temporary symptoms of hypocalcemia were noted. In contrast, no parathyroid glands were cut mistakenly in the nano-carbon group, and only one patient had transient postoperative hypocalcemia symptoms. Huang et al. (24) randomized 72 patients undergoing total thyroidectomy or total thyroidectomy with single or bilateral central lymph node dissection into the control and nano-carbon groups. The results showed hypocalcemia in 10 cases in the control group and only three in the nano-carbon group. A recent systematic review has also yielded similar results (25). Thus, carbon nanoparticles can help identify the parathyroid glands in thyroid surgery, conducive to the protection and prevention of postoperative hypoparathyroidism.
Recommendation 7: carbon nanoparticles can be used in thyroid surgery and is safe (class A).
Recommendation 8: nano-carbon negative parathyroid development technology helps identify and protect parathyroid glands during surgery and can reduce the incidence of postoperative hypoparathyroidism. Black staining of the thyroid helps identify types A1, A2 and A3 parathyroid glands, and central lymph node staining helps identify type B1 glands (class A).
Use methods and precautions
At present, most scholars recommend intraoperative injection with nano-carbon. This method can completely avoid the shortcomings of skin being dyed black, and hardly prolongs the operation length. The specific method is as below: (I) open the neck white line and the thyroid pseudocapsule, free the sternum thyroid muscle to both sides, revealing 1/3 of bilateral thyroid lobes. Be careful not to damage the integrity of the thyroid capsule, or carbon nanoparticles will spill and blacken the surrounding tissue, hindering the surgical field; (II) use a 1 mL for skin test syringe to draw carbon nanoparticles suspension to inject around the tumor tissue (upper and lower) slowly at 0.1-0.3 mL/side. Withdraw before the injection to avoid injecting vessels. For micro cancer, it is recommended to insert the syringe inside the thyroid for I° or less swollen thyroid, with approximately 0.1 mL on one side; and for enlarged thyroid of II° or above or Hashimoto’s thyroiditis, it is recommended to inject at multiple points with about 0.1 mL each. For larger tumors without apparently normal thyroid tissue, the use of nano-carbon is not recommended; (III) after the needle is withdrawn, press the injection site with gauze for around 1 min to avoid spill of nano-carbon; (IV) wait 5 min before thyroid surgery. If lateral neck lymph node dissection is to be performed first, it is recommended to do it 20 min after injection.
Some surgeons choose to inject nano-carbon preoperatively, especially endoscopic thyroid surgeons. The specific method is as follows: (I) it is recommended to perform under ultrasound guidance; (II) apply local drape using sterile ultrasound probe and the coupling agent; (III) before inserting the needle, make sure there are carbon nanoparticles outside it to avoid skin staining. Refer to the intraoperative usage for the rest; (IV) when the needle is withdrawn to the subcutaneous part, continuously retreat to withdraw under a negative pressure to avoid skin staining.
Recommendation 9: according to the surgeon’s habits, intraoperative or preoperative use of nano-carbon can be used. The preferred technique is the former and the injection volume of nano-carbon in unliateral thyroid tissue is 0.1-0.3 mL (class B).
Recommended 10: for larger tumors without apparently normal thyroid tissue, the use of nano-carbon is not recommended (class F).
Additional injection
Proper use of nano-carbon can help improve the intraoperative identification of parathyroid glands. When it is challenging to distinguish types A1 and A2 parathyroid glands from small undyed nodules on the thyroid surface, additional injection can be used to help. A small amount of nano-carbon is slowly injected into the unstained thyroid tissue around the nodules to be identified. If they are dyed black, they are thyroid nodules. Otherwise, it is highly possible that they are parathyroid glands (Figure 3).
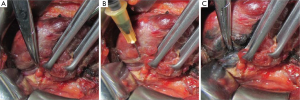
Recommendation 11: additional injection can help identify nodules on the thyroid surface and types A1 and A2 parathyroid glands (class B).
Tips and strategies to protect parathyroid glands
Surgical techniques
Meticulous capsule dissection
Meticulous capsule dissection means that the third-tier blood vessels to and from the thyroid should be treated close to the natural thyroid capsule during thyroid resection. This is helpful in retaining types A1, A2 and B parathyroid glands in situ and the blood supply.
Recommendation 12: meticulous capsule dissection is helpful in retaining types A1, A2 and B parathyroid glands in situ and the blood supply (class A).
Reservation of main blood supply to parathyroid glands in central lymph node dissection
In the central lymph node dissection, the trunk and major branches of the lower thyroid artery should be carefully dissected and retained to ensure blood supply to the upper and lower parathyroid glands (Figure 4).
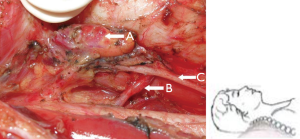
Recommendation 13: in the central lymph node dissection, the trunk and major branches of the lower thyroid artery should be retained as much as possible to ensure blood supply to the parathyroid glands (class A).
Reservations of thymus tissue in central lymph node dissection
Since types B2 and B3 parathyroid glands are closely related to the thymus, during the central lymph node dissection, if no thymic involvement is observed, the thymus tissue should be retained to avoid undesired removal of type B2 parathyroid glands and injury to blood supply to type B3 parathyroid glands.
Recommendation 14: in the central lymph node dissection, as long as the tumor does not involve the thymus, it should be retained to avoid undesired removal of type B2 parathyroid glands and injury to blood supply to type B3 parathyroid glands (class A).
Strategies of treating the lower corner area of the thyroid cartilage
The important anatomical structures in thyroid surgery are relatively concentrated in the lower corner area of the thyroid cartilage, such as the upper parathyroid glands (sometimes lower), the posterior branch of upper thyroid artery, upstream anastomotic branch of the lower thyroid artery, superior laryngeal nerve and recurrent laryngeal nerve. Thus, this is a high risk area in the surgery. Gong et al. (26) found that lymph node metastasis is rare in the lower corner area of the thyroid cartilage contralateral to the thyroid papillary carcinoma. Therefore, if the nano-carbon parathyroid negative development is used for identification and protection, for unilateral thyroid cancer, if no enlarged or positively stained lymph nodes are found in the lower corner of the contralateral thyroid cartilage, dissection of this area can be waived to reduce the incidence rate of injury to the parathyroid. This strategy is more important for repeat thyroid cancer surgery.
Recommendation 15: in the case of bilateral central lymph node dissection for unilateral thyroid cancer, if no enlarged or positively stained lymph nodes are found in the lower corner of the contralateral thyroid cartilage, dissection of this area can be waived to reduce the risk of postoperative hypoparathyroidism (class C).
Therapeutic central lymph node dissection in follicular thyroid carcinoma treatment
Since the central lymph node dissection increases the incidence of postoperative hypoparathyroidism, and follicular thyroid cancer is associated with only about 10% of lymph node metastasis, only therapeutic central lymph node dissection is done in the treatment of follicular thyroid carcinoma (14).
Recommendation 16: preventive central lymph node dissection is not performed in follicular thyroid carcinoma treatment (class A).
Proper use of advanced energy platform
Most advanced energy platforms inevitably produce heat during use, which may cause significant thermal damage to the surrounding tissue. Since parathyroid glands are sensitive to heat, operations close to them may damage the glands and their blood supply. Hence, when operating near the parathyroid glands, in the case of open surgery, a bipolar electrotome or thin suture can be used for vascular ligation; if an ultrasonic scalpel is used, the low configuration can be used while operating >3-5 mm away from the parathyroid glands and blood vessels with short continuous operation duration. When necessary, gauze with saline can be used to reduce the heat damage to the parathyroid.
Recommendation 17: rational use of advanced energy platforms can reduce intraoperative damage to parathyroid glands and blood supply (class C).
Careful search for parathyroid glands in specimens
Before sending the dissected thyroid and central tissue for pathological examination, routine caution should be given to identify parathyroid glands mistakenly cut. Since type A3 parathyroid glands are present in the thyroid tissue and cannot be identified or retained in situ, it is recommended that, when conditions permit, the thyroid tissue is dissected at an interval of 1-2 mm from front to rear longitudinally while paying attention to retain the backside continuity of about 1 mm thick to facilitate retention of the intact anatomical structure. Careful observation is needed of the presence or absence of type A3 parathyroid glands each cross section. During central tissue dissection, it is also needed to carefully check if any parathyroid glands are mistakenly cut. In general, it is required for fat tissue to be dissected to translucent to avoid missed parathyroid glands to the maximum extent.
Recommendation 18: it is necessary to routinely look for mistakenly cut parathyroid glands during surgery (class B).
Parathyroid autotransplantation
Parathyroid autograft transplant means to transplant the parathyroid glands that cannot be retained in situ or is mistakenly removed to other specific sites after pathological confirmation (1-2 mm specimen). Studies have shown that as long as the correct method of autologous transplantation is used, these grafts can survive and play a physiological function. Several studies have confirmed that in conventional thyroidectomy, 1 to 2 parathyroid autografts can almost avoid severe postoperative permanent hypoparathyroidism (27,28).
Particle entrapment
This is to cut parathyroid glands into particulates of <1 mm, decentralize and put them into the sternocleidomastoid muscle (“pocket”), and marked with non-absorbable sutures to help future recognition in the case of repeat operation. Caution should be made to completely stop the bleeding of the muscles to avoid hematoma, otherwise it may affect the survival of the implanted parathyroid tissue. In the event of serious localized invasion of the tumor and there is a high probability of recurrence, it is recommended to transplant the parathyroid tissue to the forearm or deltoid muscles. It should be noted that to improve graft survival, close placement of the parathyroid particles should be avoided. Multiple “pockets” (in the same or other muscles) can be made to reduce the risk of graft failure at a single site.
Homogenization injection
In homogenization injection, the parathyroid tissue is cut into pieces and fused into a syringe to inject into the forearm muscles at approximately 1 mL. The depth of injection should be carefully tapered by withdrawing while injecting to avoid concentrated grafts in one site, affecting their survival. The method is particularly suitable for use in a completely endoscopic thyroid surgery.
The parathyroid graft sites also include other muscles (such as the chest, trapezius) and subcutaneous tissue (such as the forearm, abdominal wall, etc.). In the thyroid surgery notes, the number and locations of parathyroid glands found, whether autologous transplant is conducted, and transplant number and locations should be recorded in detail to reduce damage to the parathyroid glands in repeat operations.
Recommendation 19: autologous transplantation of parathyroid glands mistakenly cut or unmaintainable in situ can effectively reduce the incidence of severe postoperative permanent hypoparathyroidism (class A).
Postoperative management
Postoperative serum PTH and calcium detection
Serum PTH and calcium levels are measured after day 1, day 3 and 1 month following the surgery to evaluate parathyroid functions. If serum PTH and calcium levels are still lower than normal or low calcium symptoms are present after a month, follow-up review of the serum PTH and calcium levels should be continued at a frequency depending on the circumstances.
Postoperative calcium supplementation
It is recommended to provide routine intravenous calcium after thyroid surgery, usually using 10% gluconate. A gradual transition to oral calcium supplementation or discontinuation is needed according to the patient’s clinical symptoms, serum PTH and calcium levels. Oral calcium can be supplemented by vitamin D while appropriate.
Recommendation 20: serum PTH and calcium levels should be monitored after thyroid surgery, and calcium supplementation is needed based on clinical symptoms and findings (class B).
Conclusions
Under the general principles of “1+ X”, the use of the meticulous capsule dissection technology, proper application of nano-carbon parathyroid negative development protection, careful identification of mistakenly cut parathyroid glands following detailed recognition and protection, autotransplant, postoperative management and other aspects are key to effective prevention of severe postoperative permanent hypoparathyroidism.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Jung KW, Won YJ, Kong HJ, et al. Cancer statistics in Korea: incidence, mortality, survival, and prevalence in 2012. Cancer Res Treat 2015;47:127-41. [PubMed]
- Liu YQ, Zhang SQ, Chen WQ, et al. Trend of incidence and mortality on thyroid cancer in China during 2003 - 2007. Zhonghua Liu Xing Bing Xue Za Zhi 2012;33:1044-8. [PubMed]
- Grebe SK, Hay ID. Thyroid cancer nodal metastases: biologic significance and therapeutic considerations. Surg Oncol Clin N Am 1996;5:43-63. [PubMed]
- Mazzaferri EL. Management of a solitary thyroid nodule. N Engl J Med 1993;328:553-9. [PubMed]
- Scheumann GF, Gimm O, Wegener G, et al. Prognostic significance and surgical management of locoregional lymph node metastases in papillary thyroid cancer. World J Surg 1994;18:559-67; discussion 567-8. [PubMed]
- Qubain SW, Nakano S, Baba M, et al. Distribution of lymph node micrometastasis in pN0 well-differentiated thyroid carcinoma. Surgery 2002;131:249-56. [PubMed]
- Gimm O, Rath FW, Dralle H. Pattern of lymph node metastases in papillary thyroid carcinoma. Br J Surg 1998;85:252-4. [PubMed]
- Henry JF, Gramatica L, Denizot A, et al. Morbidity of prophylactic lymph node dissection in the central neck area in patients with papillary thyroid carcinoma. Langenbecks Arch Surg 1998;383:167-9. [PubMed]
- Lee YS, Kim SW, Kim SW, et al. Extent of routine central lymph node dissection with small papillary thyroid carcinoma. World J Surg 2007;31:1954-9. [PubMed]
- Pereira JA, Jimeno J, Miquel J, et al. Nodal yield, morbidity, and recurrence after central neck dissection for papillary thyroid carcinoma. Surgery 2005;138:1095-100, discussion 1100-1. [PubMed]
- Sywak M, Cornford L, Roach P, et al. Routine ipsilateral level VI lymphadenectomy reduces postoperative thyroglobulin levels in papillary thyroid cancer. Surgery 2006;140:1000-5; discussion 1005-7. [PubMed]
- Ito Y, Tomoda C, Uruno T, et al. Clinical significance of metastasis to the central compartment from papillary microcarcinoma of the thyroid. World J Surg 2006;30:91-9. [PubMed]
- Goropoulos A, Karamoshos K, Christodoulou A, et al. Value of the cervical compartments in the surgical treatment of papillary thyroid carcinoma. World J Surg 2004;28:1275-81. [PubMed]
- American Thyroid Association (ATA) Guidelines Taskforce on Thyroid Nodules and Differentiated Thyroid Cancer. Revised American Thyroid Association management guidelines for patients with thyroid nodules and differentiated thyroid cancer. Thyroid 2009;19:1167-214. [PubMed]
- Cheah WK, Arici C, Ituarte PH, et al. Complications of neck dissection for thyroid cancer. World J Surg 2002;26:1013-6. [PubMed]
- Giordano D, Valcavi R, Thompson GB, et al. Complications of central neck dissection in patients with papillary thyroid carcinoma: results of a study on 1087 patients and review of the literature. Thyroid 2012;22:911-7. [PubMed]
- Wang X, Li Y. Anatomical study on the parathyroid glands of Chinese adults. J Ningxia Medical College 2003;25:183-5.
- Li ZH, Zhu JQ, Wei T, et al. Feature and clinical significance of parathyroid disposition in human body (anatomical research report of 50 cases). Chin J Bases Clin General Surg 2008;15:311-3.
- Song CM, Jung JH, Ji YB, et al. Relationship between hypoparathyroidism and the number of parathyroid glands preserved during thyroidectomy. World J Surg Oncol 2014;12:200. [PubMed]
- Yang F, Jin C, Yang D, et al. Magnetic functionalised carbon nanotubes as drug vehicles for cancer lymph node metastasis treatment. Eur J Cancer 2011;47:1873-82. [PubMed]
- Hagiwara A, Takahashi T, Sawai K, et al. Lymph nodal vital staining with newer carbon particle suspensions compared with India ink: experimental and clinical observations. Lymphology 1992;25:84-9. [PubMed]
- Zhu J, Wang Z, Wei T, et al. Application of Lymphatic Mapping to Recognize and Protect Negative Stained Parathyroid in Thyroid Carcinoma Surgery by Using Carbon Nanoparticles. Chin J Bases Clin General Surg 2013;20:992-4.
- Zeng Y, Qian J, Cheng R, et al. Protective effect of lymphatic tracer on parathyroid glands in lymph node dissection in thyroid carcinoma. Chongqing Medicine 2012;41:1076-7.
- Huang K, Luo D, Huang M, et al. Protection of parathyroid function using carbon nanoparticles during thyroid surgery. Otolaryngol Head Neck Surg 2013;149:845-50. [PubMed]
- Li Y, Jian WH, Guo ZM, et al. A Meta-analysis of Carbon Nanoparticles for Identifying Lymph Nodes and Protecting Parathyroid Glands during Surgery. Otolaryngol Head Neck Surg 2015;152:1007-16. [PubMed]
- Gong YP, Gong RX, Zhu JQ, et al. Clinical research strategy for cN0 thyroid papillary carcinoma central lymph node dissection. Zhonghua Wai Ke Za Zhi 2013;51:1081-4. [PubMed]
- Ahmed N, Aurangzeb M, Muslim M, et al. Routine parathyroid autotransplantation during total thyroidectomy: a procedure with predictable outcome. J Pak Med Assoc 2013;63:190-3. [PubMed]
- Testini M, Rosato L, Avenia N, et al. The impact of single parathyroid gland autotransplantation during thyroid surgery on postoperative hypoparathyroidism: a multicenter study. Transplant Proc 2007;39:225-30. [PubMed]