Novel agents and treatment techniques to enhance radiotherapeutic outcomes in carcinoma of the uterine cervix
Introduction
Concurrent chemo-radiotherapy (CCRT) with cisplatin followed by brachytherapy has become the standard treatment for locally advanced (stage IB-IVA) cervical carcinoma (LACC) worldwide. The 5-year survival with this approach for LACC has reached a plateau (1) of 50–60% and this also comes at the cost of high toxicities (2). Modern techniques of radiation treatment has decreased the morbidities but has helped little in bringing any change in survival (3). Protocols other than CCRT like induction chemotherapy (4) or consolidative chemotherapy (5) have shown inconclusive advantage over CCRT alone and needs further exploration in well-designed trials. Thus, incorporation of novel agents, modification of existing protocols, intensification of regimens, and integration of local therapies with CCRT programs may be the way forward to improve and optimize the outcome of LACC patients. The present article discusses on the present evidence and future direction towards amalgamation of translational and clinical research in this arena and aims to ignite perspectives for the design of further clinical trials.
Modulation of existing CTRT protocols
Platinum based regimens
CCRT with weekly cisplatin remains the most widely used regimen worldwide and is the present gold standard to which other regimens should be compared (6). Three weekly regimens compared to weekly regimens suggested higher toxicity and delay of the treatment course with the former regimen (7). Most of the trials which formed the basis of current CCRT regimen used a combination of cisplatin and fluorouracil with or without hydroxyurea (1). Results of a randomized trial by Kim et al. (8) showed non-inferiority of weekly cisplatin to cisplatin plus fluorouracil combination; 4-year overall survival was 67% with cisplatin alone vs. 70% with combination and higher toxicity with the combination regimens (grade 3/4 hematological toxicity 43% vs. 26%; P=0.037).
Carboplatin is thought to be an active radio-sensitizer (9) and the side effect profiles may tempt clinicians to substitute it with cisplatin in certain situations like elderly patients, patient with renal dysfunction or with multiple co-morbidities (10). Katanyoo et al. (11) reported results of 148 patients of LACC treated with weekly concurrent carboplatin (AUC 2 or 100 mg/m2) and found 5-year overall survival rates of 63.5% without any grade 3/4 toxicities. Au-Yeung et al. (12) in their retrospective audit of 442 patients of LACC showed inferior outcomes with weekly carboplatin as compared to cisplatin in terms of both disease free and overall survival. However, in the absence of a head to head comparative trial evaluating these regimens, the controversy remains unsettled.
Nedaplatin (an analogue of cisplatin) has been tried in a phase II study by Yokoyama et al. (13); 45 patients treated with concurrent nedaplatin showed a better toxicity profile as compared to cisplatin based regimens (grade 3 gastrointestinal toxicity of 4.4%; grade 4 hematological toxicity of 6.7%). Niibe et al. (14) reported 80% complete response rates for LACC with this approach in their study on 10 patients. A phase II study by Zhang et al. (15) also found combination of nedaplatin and paclitaxel to be tolerable (grade 3 or higher hematological toxicity of 10.9%) and effective with 2-year overall survival rate of 93% in 34 patients of LACC.
Taxane based regimens
The survival advantage with concurrent regimens has generated great enthusiasm in the search for newer regimens, drugs and schedules. Rao et al. (16) in a phase I clinical trial demonstrated feasibility and activeness of weekly paclitaxel/carboplatin with concomitant radiation. The maximum tolerated dose for paclitaxel was 50 mg/m2 and for carboplatin was AUC 2.5. Two-year overall survival and progression free survival was 86% and 80% respectively. Combination of paclitaxel and cisplatin (17) or paclitaxel and vinorelbine (18) in order to further improve the effectiveness was met with higher toxicity and is not recommended now. Geara et al. (19) reported results of randomized trial comparing weekly cisplatin (40 mg/m2) to paclitaxel (50 mg/m2) concomitant with radiotherapy in LACC. Five-year overall survival was 54% with cisplatin vs. 43% with paclitaxel and the difference was non-significant.
Topoisomerase based regimens
Extrapolating from the benefit of combination of topotecan and cisplatin in metastatic and recurrent cervical carcinoma, Gatcliffe et al. (20) studied the role of this combination for radiosensitization in radical treatment of locally advanced cervical carcinoma. A weekly dose of 30 mg/m2 of cisplatin and 2 mg/m2 of topotecan were found to be tolerable and feasible with acceptable toxicity. In another study by Rose et al. (21), the tolerable doe levels were found to be 30 mg/m2 of cisplatin and 0.5 mg/m2 of topotecan. Fabbro et al. (22) defined the phase II dose of another topoisomerase inhibitor (Irinotecan) concomitant with cisplatin and radiotherapy. The doses suggested were cisplatin 20 mg/m2 and irinotecan 35 mg/m2 weekly. These studies have reported feasibility and have defined drug doses for these regimens. Response rates have ranged from 70–90% and survival outcomes have not been reported elaborately.
Gemcitabine based regimens
Zarbá et al. (23) showed encouraging complete response rates of 88.8% with concurrent cisplatin and gemcitabine with an acceptable toxicity (grade 3/4) rate of <20%. Drawing from these results, a phase III trial (24) evaluated the role of concurrent cisplatin plus gemcitabine vs. standard cisplatin based CCRT. The experimental arm also used 2 cycles of adjuvant chemotherapy with the same drug combination. The authors reported a superior overall survival (HR, 0.68; 95% CI, 0.49–0.95; P=0.0224) in the intervention group which came at the cost of overwhelming grade 3/4 toxicity of 71.9% compared with 23.9% in the standard arm. The results of this study are unique in a way that the combination has shown survival advantage over the ‘gold standard’ of weekly cisplatin regimen. However, it remains unclear whether the benefit came from the novel chemo-radiotherapy combination or the addition of adjuvant chemotherapy.
Summary and future directions
Concurrent chemo-radiation with weekly cisplatin remains the gold standard as of now for LACC. The following drug combination and regimens needs to be tested against weekly cisplatin in well designed phase III trials for better defining their role as well as optimization of existing CCRT protocol:
- Concurrent gemcitabine and cisplatin;
- Concurrent paclitaxel with or without platinum;
- Concurrent carboplatin in subset of patients;
- Concurrent nedaplatin with or without taxane;
- Concurrent topotecan/irinotecan with or without platinum.
Molecular targeted and other novel agents with CCRT regimens
Agents tried in phase I/II studies
Commensurate with use of molecular targeted agents in other cancers, several drugs and agents are being evaluated for LACC; however, most of these are for metastatic and refractory cases. Limited studies have evaluated the incorporation of these in the radical CCRT protocols. Bevacizumab (25), erlotinib (26), cetuximab (27) and cyclo-oxygenase inhibitor celecoxib (28) have been all tried with CCRT regimens and found to be well tolerated and feasible in phase I/II studies.
Novel molecular pathway
Newer molecular pathways are being explored to find out the mechanism of resistance as well as targets of radiosensitization. Activation of PI3K/Akt/COX-2 pathway has been implicated in induction of resistance to radiation in cervical cancer HeLa cells (29). In a proof of principle study, it was later demonstrated that inhibition of phosphoinositide-3-kinase inhibition (by PI3K inhibitor-LY294002) leads to increased radio-sensitivity in vivo (30,31). The central mechanism of action of this drug was by inhibition of DNA-PK (DNA protein kinase) causing inhibition of DNA double stranded break repair (32). There is an urgent need of phase I/II trials for further elucidating and defining the role of PI3K inhibition in cervical cancer (33). Kunos et al. (34) investigated the role of M1, M2, M2b ribonucleotide reductase (RNR) subunit levels in 18 cervical cancer specimens and found over expression of M1 and M2b subunits to be associated with poor response to chemo-radiotherapy and to decreased disease free survival. In a subsequent study, the authors (35) used 3-aminopyridine-2-carboxaldehyde thiosemicarbazone (3-AP; an inhibitor of RNR) along with cisplatin and concurrent radiation in 22 patients of LACC. The combination was very well tolerated with clinical response of 96%. The encouraging results needs to further validated in randomized trial before clinical applicability.
Non-cytotoxic agents as radiosensitizers
Some of the drugs routinely used for other diseases have been found to have molecular signaling pathways which could be harnessed in cervical carcinoma. Hydralazine (36) (an anti-hypertensive agent) and magnesium valproate (37) (an anti-epileptic agent) has been found to be effective modifiers of radiation and are well tolerated with CCRT. troglitazone (a peroxisome proliferator-activated receptor agonist and anti-diabetic agent) has been found to increase radiation sensitivity of cervical cancer cells (HeLa cell lines) via G1 arrest (38). Pentoxifylline (a methylxantine drug used for circulatory disease) have been found to increase sensitivity of cervical cancer cells to cisplatin induced damage by inhibition of NF-κB pathway (39). Epigenetic modification is yet another way of potentiating the cytotoxicity as well as radiosensitivity of CCRT regimens. This may require further testing in vivo studies as well as phase I testing before reaching at any further conclusions.
Human papilloma virus (HPV) and radio-therapeutic outcomes
HPV infection is associated with >90% of the cervical carcinoma and HPV 16/18 accounts for >70% of all cervical cancers worldwide (40). Recent studies have suggested increased radiosensitivity of HPV related head neck cancers (accounting for 5–20% of all head and neck cancers) to radiotherapy and chemotherapy and several strategies and trials towards de-escalation of treatment intensity in these subset of patients (41). A similar trend although premature is emerging for cervical carcinoma as well. Patients with high HPV titers at baseline (>1,000 relative light units) showed higher complete response rates as well as better overall survival as compared to those with low titers in the study by Datta et al. (42,43). A reduction of these values to 99.5% of the baseline values also predicted better survival in this study (42) and persistence of HPV infection leads to increased recurrence rates (43,44). HPV-16 associated oncoproteins E6 and E7 could also be potential targets of therapy. Sima et al. (45) showed increased apoptosis and senescence of transfected cervical carcinoma cells after treatment with antisense RNA and this approach needs further testing.
Summary and future directions
The landscape of combination of molecular targeting with CCRT regimen is rapidly evolving. The following warrants further research in this regard:
- Phase III studies of bevacizumab/erlotinib/celecoxib/cetuximab with CCRT;
- Phase I/II studies of PI3K inhibitor, RNR inhibitor, pentoxifylline and hydralazine with CCRT;
- HPV titer adapted treatment protocols (intensification for patients with less reduction of titers after CCRT).
Local therapies in combination with CTRT regimens
Hyperthermia and CTRT regimens
Combination of radiotherapy with hyperthermia remains a controversial and debated topic. Different mechanism of actions and toxicity profiles prompted interest in combining the two treatment modalities. Studies in other tumor sites like glioblastoma multiforme (46) and esophageal cancers (47) demonstrated improvement in local response rates and survival when thermo-radiotherapy was compared to radiotherapy alone. Results of this strategy is inconclusive for carcinoma cervix; while a randomized (48) International Atomic Energy Agency (IAEA) study failed to show benefit of thermo-radiotherapy over radiotherapy alone, other studies showed improvement in both local control as well as overall survival (49,50). A Cochrane analysis (51) based on the present available evidence suggested a benefit of thermo-radiotherapy approach over radiotherapy in terms of local control as well as overall survival without increase in grade 3 to 4 toxicity. One limitation of the older studies was the available device for the delivery of hyperthermia (48) and newer methods of catheter based ultrasound devices (52) may help in better and more conformal delivery of hyperthermia and thus further improving the therapeutic ratio. Despite the scientific rationale and proven results of hyperthermia, non-availability of the facility has restricted its use to limited number of centres worldwide. Time has also become ripe to propose trial designs comparing thermo-radiotherapy to chemo-radiotherapy in locally advanced cervical carcinoma.
Direct local delivery of agents
Direct local delivery of the radiation source, chemotherapeutic or targeted agents appear to be an innovative and novel method of cytotoxicity, particularly in cervix where local boost plays a vital role in the management. However, these methods have several challenges viz. unpredictable and sometimes high systemic absorption of locally delivered drugs, diffusion of the agents outside the interstitial spaces of tumor, backflow of the agents etc. Kim et al. (53) recently published their report on the use of micron-size radiotherapy source using a temperature sensitive hydrogel (RT-GEL). This consists of In-111 mixed with RT-GEL. Once injected in to the tumor, the solution converts in to a gel and is retained locally in the tumor tissue and delivers local radiation from the In-111 source. They also demonstrated no backflow with this technique, minimal systemic absorption and demonstratable cytotoxic effect on human breast tumor cells in animal models as compared to the control of In-111 admixed with saline. The initial results appear promising and definitely deserve testing in cervical cancer cells as well. Hodge et al. (54) reported local delivery of gemcitabine in patients with gynecological malignancy using a novel cervical delivery device (CerviPrepTM). Cervical tissue, samples from the uterine vein as well as plasma was collected from 17 patients undergoing hysterectomy and they detected pharmacologically relevant concentration of gemcitabine in the cervical tissue. Even though concentrations of gemcitabine alone might not be enough for radio-sensitizing effect, combining this with low doses of cisplatin might be a step towards optimal radio-sensitization effect along with minimal systemic toxicity.
Nano-medicines as radio-sensitizing strategy
Gold nano-particles (GNPs) have been found to cause radiosensitization with kilovoltage (kV) radiation (55). The effect has predominantly been dedicated to increased photon absorption in high Z material and dominant role of photoelectric effect at kV radiation. Monte Carlo studies predicted less radiosensitization effect of GNP in the megavoltage (MV) range (56). However, the predictions are far from simple and assume only physical interaction of radiation with GNPs. Recent studies have found out additional mechanism of cytotoxicity like reactive oxygen species mediated, arrest of cytokinesis, apoptosis, inhibition of DNA repair etc. (57,58). Jain et al. (59) in their study on breast cancer cells demonstrated radiation sensitizer enhancement ratio of 1.29 and 1.16 respectively with 6 and 15 MV radiation respectively. Higher concentration of GNPs may be required in order to achieve higher dose enhancement factors; however this may come at the cost of increased toxicities. Combination of colloid GNPs with chemotherapeutic agents may be a novel mode of radiosensitization and needs testing in clinical trials. In fact, GNPs have already entered phase I trials in advanced cancer patients. Libutti et al. (60) reported results of pharmacokinetics study of CYT-6091 (constructed by combining recombinant human tumor necrosis factor alpha and thiolyated polyethylene glycol to the surface of colloidal GNPs) and found this nano-medicine to be well tolerated without serious adverse events.
Summary and future directions
The combination of local therapy with CCRT regimen appears promising. Although the results are premature, further testing definitely holds a ray of hope.
- Phase III trials evaluating thermo-radiotherapy to CCRT;
- Phase I/II studies of local delivery of cytotoxic agents in combination with low systemic dose of concurrent chemotherapy;
- In-vitro, In-vivo and cell line studies on application of nano-medicine with CCRT.
Palliation with CTRT regimens
Hypofractionated radiotherapy has been used for palliation of symptoms in locally advanced cervical carcinoma. A systemic review (61) in 2011 suggested paucity of existing literature on the optimal fractionation schedule for the same and need of future trials. Even in absence of level I evidence, most centres would prefer a fractionation schedule of 20–30 Gray in 5–10 fractions over 1–2 weeks (62). Radiation Therapy Oncology Group (RTOG) adopted a 10 Gray monthly dose with misonidazole in a quest to achieve a convenient and superior palliation (63), the study however was terminated in view of severe late gastrointestinal complications. There is an unmet need of research for this subset of patients. Study by Lagrange et al. (64) seems interesting in this regard; the authors evaluated the role of short infusion of 5 mg cisplatin for 5 consecutive days concomitant with radiation and found tumoral concentration of platinum to be in the range of radio-sensitization. Combining low daily dose of cisplatin with modest doses of radiation (20-25 Gray) may be an effective method of achieving palliation.
Table 1 summarizes the present status and future research directions on the use of novel agents and treatment techniques.
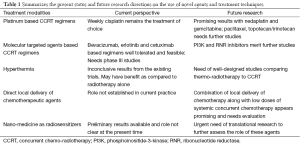
Full table
Conclusions
A number of approaches have appeared; initially as ripples and later as large promising waves for optimization of CCRT protocols in the management of carcinoma of uterine cervix. While many of these are in preliminary phases of investigations, some of these warrant immediate testing in phase III randomized trials. Molecular targeted agents, local delivery of cytotoxic agents and nano-medicines may become the frontline of management in the times to come and a more intricate co-ordination of translational and clinical research may bring overwhelming results. The enthusiasm and optimism of these novel agents and treatment techniques must be weighed against the widespread applicability particularly in resource constraint setting. Future studies should also focus on quality of life issues and cost-effectiveness analysis of these novel interventions. Nevertheless, the time perhaps has arrived for a breakthrough in the stagnant outcomes of carcinoma cervix with these preliminary and immature yet promising approaches.
Acknowledgements
None.
Footnote
Conflicts of Interest: The author has no conflicts of interest to declare.
References
- Green JA, Kirwan JM, Tierney JF, et al. Survival and recurrence after concomitant chemotherapy and radiotherapy for cancer of the uterine cervix: a systematic review and meta-analysis. Lancet 2001;358:781-6. [PubMed]
- Kirwan JM, Symonds P, Green JA, et al. A systematic review of acute and late toxicity of concomitant chemoradiation for cervical cancer. Radiother Oncol 2003;68:217-26. [PubMed]
- Gandhi AK, Sharma DN, Rath GK, et al. Early clinical outcomes and toxicity of intensity modulated versus conventional pelvic radiation therapy for locally advanced cervix carcinoma: a prospective randomized study. Int J Radiat Oncol Biol Phys 2013;87:542-8. [PubMed]
- Duenas-Gonzalez A, Lopez-Graniel C, Gonzalez-Enciso A, et al. Concomitant chemoradiation versus neoadjuvant chemotherapy in locally advanced cervical carcinoma: results from two consecutive phase II studies. Ann Oncol 2002;13:1212-9. [PubMed]
- Tangjitgamol S, Katanyoo K, Laopaiboon M, et al. Adjuvant chemotherapy after concurrent chemoradiation for locally advanced cervical cancer. Cochrane Database Syst Rev 2014;12:CD010401. [PubMed]
- Gandhi AK, Sharma DN, Rath GK. Concurrent chemoradiation for carcinoma of cervix: what lies beyond? J Cancer Res Ther 2014;10:227-8. [PubMed]
- Chumworathayi B, Suprasert P, Charoenkwan K, et al. Weekly versus three-weekly cisplatin as an adjunct to radiation therapy in high-risk stage I-IIA cervical cancer after surgery: a randomized comparison of treatment compliance. J Med Assoc Thai 2005;88:1483-92. [PubMed]
- Kim YS, Shin SS, Nam JH, et al. Prospective randomized comparison of monthly fluorouracil and cisplatin versus weekly cisplatin concurrent with pelvic radiotherapy and high-dose rate brachytherapy for locally advanced cervical cancer. Gynecol Oncol 2008;108:195-200. [PubMed]
- Micheletti E, La Face B, Bianchi E, et al. Continuous infusion of carboplatin during conventional radiotherapy treatment in advanced squamous carcinoma of the cervix uteri IIB-IIIB (UICC). A phase I/II and pharmacokinetic study. Am J Clin Oncol 1997;20:613-20. [PubMed]
- Cetina L, Garcia-Arias A, Uribe Mde J, et al. Concurrent chemoradiation with carboplatin for elderly, diabetic and hypertensive patients with locally advanced cervical cancer. Eur J Gynaecol Oncol 2008;29:608-12. [PubMed]
- Katanyoo K, Tangjitgamol S, Chongthanakorn M, et al. Treatment outcomes of concurrent weekly carboplatin with radiation therapy in locally advanced cervical cancer patients. Gynecol Oncol 2011;123:571-6. [PubMed]
- Au-Yeung G, Mileshkin L, Bernshaw DM, et al. Radiation with cisplatin or carboplatin for locally advanced cervix cancer: the experience of a tertiary cancer centre. J Med Imaging Radiat Oncol 2013;57:97-104. [PubMed]
- Yokoyama Y, Takano T, Nakahara K, et al. A phase II multicenter trial of concurrent chemoradiotherapy with weekly nedaplatin in advanced uterine cervical carcinoma: Tohoku Gynecologic Cancer Unit Study. Oncol Rep 2008;19:1551-6. [PubMed]
- Niibe Y, Tsunoda S, Jobo T, et al. Phase II study of radiation therapy combined with weekly nedaplatin in locally advanced uterine cervical carcinoma (LAUCC): Kitasato Gynecologic Radiation Oncology Group (KGROG 0501)--initial analysis. Eur J Gynaecol Oncol 2008;29:222-4. [PubMed]
- Zhang MQ, Liu SP, Wang XE. Concurrent chemoradiotherapy with paclitaxel and nedaplatin followed by consolidation chemotherapy in locally advanced squamous cell carcinoma of the uterine cervix: preliminary results of a phase II study. Int J Radiat Oncol Biol Phys 2010;78:821-7. [PubMed]
- Rao GG, Rogers P, Drake RD, et al. Phase I clinical trial of weekly paclitaxel, weekly carboplatin, and concurrent radiotherapy for primary cervical cancer. Gynecol Oncol 2005;96:168-72. [PubMed]
- Martínez-Monge R, Gaztañaga M, Aramendía JM, et al. A phase II trial of less than 7 weeks of concomitant cisplatin-paclitaxel chemoradiation in locally advanced cervical cancer. Int J Gynecol Cancer 2010;20:133-40. [PubMed]
- Mundt AJ, Rotmensch J, Waggoner SE, et al. Phase I trial of concomitant vinorelbine, paclitaxel, and pelvic irradiation in cervical carcinoma and other advanced pelvic malignancies. Gynecol Oncol 2001;82:333-7. [PubMed]
- Geara FB, Shamseddine A, Khalil A, et al. A phase II randomized trial comparing radiotherapy with concurrent weekly cisplatin or weekly paclitaxel in patients with advanced cervical cancer. Radiat Oncol 2010;5:84. [PubMed]
- Gatcliffe TA, Tewari KS, Shah A, et al. A feasibility study of topotecan with standard-dose cisplatin and concurrent primary radiation therapy in locally advanced cervical cancer. Gynecol Oncol 2009;112:85-9. [PubMed]
- Rose PG, Sill MW, McMeekin DS, et al. A phase I study of concurrent weekly topotecan and cisplatin chemotherapy with whole pelvic radiation therapy in locally advanced cervical cancer: a gynecologic oncology group study. Gynecol Oncol 2012;125:158-62. [PubMed]
- Fabbro M, Gladieff L, Guichard F, et al. Phase I study of irinotecan and cisplatin in combination with pelvic radiotherapy in the treatment of locally advanced cervical cancer: A GINECO trial. Gynecol Oncol 2010;117:276-80. [PubMed]
- Zarbá JJ, Jaremtchuk AV, Gonzalez Jazey P, et al. A phase I-II study of weekly cisplatin and gemcitabine with concurrent radiotherapy in locally advanced cervical carcinoma. Ann Oncol 2003;14:1285-90. [PubMed]
- Dueñas-González A, Zarbá JJ, Patel F, et al. Phase III, open-label, randomized study comparing concurrent gemcitabine plus cisplatin and radiation followed by adjuvant gemcitabine and cisplatin versus concurrent cisplatin and radiation in patients with stage IIB to IVA carcinoma of the cervix. J Clin Oncol 2011;29:1678-85. [PubMed]
- Schefter TE, Winter K, Kwon JS, et al. A phase II study of bevacizumab in combination with definitive radiotherapy and cisplatin chemotherapy in untreated patients with locally advanced cervical carcinoma: preliminary results of RTOG 0417. Int J Radiat Oncol Biol Phys 2012;83:1179-84. [PubMed]
- Nogueira-Rodrigues A, do Carmo CC, Viegas C, et al. Phase I trial of erlotinib combined with cisplatin and radiotherapy for patients with locally advanced cervical squamous cell cancer. Clin Cancer Res 2008;14:6324-9. [PubMed]
- Moore KN, Sill MW, Miller DS, et al. A phase I trial of tailored radiation therapy with concomitant cetuximab and cisplatin in the treatment of patients with cervical cancer: A gynecologic oncology group study. Gynecol Oncol 2012;127:456-61. [PubMed]
- Gaffney DK, Winter K, Dicker AP, et al. A Phase II study of acute toxicity for Celebrex (celecoxib) and chemoradiation in patients with locally advanced cervical cancer: primary endpoint analysis of RTOG 0128. Int J Radiat Oncol Biol Phys 2007;67:104-9. [PubMed]
- Xia S, Zhao Y, Yu S, et al. Activated PI3K/Akt/COX-2 pathway induces resistance to radiation in human cervical cancer HeLa cells. Cancer Biother Radiopharm 2010;25:317-23. [PubMed]
- Liu Y, Cui B, Qiao Y, et al. Phosphoinositide-3-kinase inhibition enhances radiosensitization of cervical cancer in vivo. Int J Gynecol Cancer 2011;21:100-5. [PubMed]
- Lee CM, Fuhrman CB, Planelles V, et al. Phosphatidylinositol 3-kinase inhibition by LY294002 radiosensitizes human cervical cancer cell lines. Clin Cancer Res 2006;12:250-6. [PubMed]
- Fuhrman CB, Kilgore J, LaCoursiere YD, et al. Radiosensitization of cervical cancer cells via double-strand DNA break repair inhibition. Gynecol Oncol 2008;110:93-8. [PubMed]
- Wu J, Chen C, Zhao KN. Phosphatidylinositol 3-kinase signaling as a therapeutic target for cervical cancer. Curr Cancer Drug Targets 2013;13:143-56. [PubMed]
- Kunos CA, Radivoyevitch T, Kresak A, et al. Elevated ribonucleotide reductase levels associate with suppressed radiochemotherapy response in human cervical cancers. Int J Gynecol Cancer 2012;22:1463-9. [PubMed]
- Kunos CA, Radivoyevitch T, Waggoner S, et al. Radiochemotherapy plus 3-aminopyridine-2-carboxaldehyde thiosemicarbazone (3-AP, NSC #663249) in advanced-stage cervical and vaginal cancers. Gynecol Oncol 2013;130:75-80. [PubMed]
- Candelaria M, Cetina L, Pérez-Cárdenas E, et al. Epigenetic therapy and cisplatin chemoradiation in FIGO stage IIIB cervical cancer. Eur J Gynaecol Oncol 2010;31:386-91. [PubMed]
- Chavez-Blanco A, Segura-Pacheco B, Perez-Cardenas E, et al. Histone acetylation and histone deacetylase activity of magnesium valproate in tumor and peripheral blood of patients with cervical cancer. A phase I study. Mol Cancer 2005;4:22. [PubMed]
- An Z, Liu X, Song H, et al. Effect of troglitazone on radiation sensitivity in cervix cancer cells. Radiat Oncol J 2012;30:78-87. [PubMed]
- Hernandez-Flores G, Ortiz-Lazareno PC, Lerma-Diaz JM, et al. Pentoxifylline sensitizes human cervical tumor cells to cisplatin-induced apoptosis by suppressing NF-kappa B and decreased cell senescence. BMC Cancer 2011;11:483. [PubMed]
- Castellsagué X. Natural history and epidemiology of HPV infection and cervical cancer. Gynecol Oncol 2008;110:S4-7. [PubMed]
- Boscolo-Rizzo P, Del Mistro A, Bussu F, et al. New insights into human papillomavirus-associated head and neck squamous cell carcinoma. Acta Otorhinolaryngol Ital 2013;33:77-87. [PubMed]
- Datta NR, Kumar P, Singh S, et al. Does pretreatment human papillomavirus (HPV) titers predict radiation response and survival outcomes in cancer cervix?--a pilot study. Gynecol Oncol 2006;103:100-5. [PubMed]
- Datta NR, Singh S, Kumar P, et al. Human papillomavirus confers radiosensitivity in cancer cervix: a hypothesis toward a possible restoration of apoptotic pathways based on clinical outcomes. Future Oncol 2015;11:1363-71. [PubMed]
- Nagai Y, Toma T, Moromizato H, et al. Persistence of human papillomavirus infection as a predictor for recurrence in carcinoma of the cervix after radiotherapy. Am J Obstet Gynecol 2004;191:1907-13. [PubMed]
- Sima N, Wang S, Wang W, et al. Antisense targeting human papillomavirus type 16 E6 and E7 genes contributes to apoptosis and senescence in SiHa cervical carcinoma cells. Gynecol Oncol 2007;106:299-304. [PubMed]
- Sneed PK, Stauffer PR, McDermott MW, et al. Survival benefit of hyperthermia in a prospective randomized trial of brachytherapy boost +/- hyperthermia for glioblastoma multiforme. Int J Radiat Oncol Biol Phys 1998;40:287-95. [PubMed]
- Kitamura K, Kuwano H, Watanabe M, et al. Prospective randomized study of hyperthermia combined with chemoradiotherapy for esophageal carcinoma. J Surg Oncol 1995;60:55-8. [PubMed]
- Vasanthan A, Mitsumori M, Park JH, et al. Regional hyperthermia combined with radiotherapy for uterine cervical cancers: a multi-institutional prospective randomized trial of the international atomic energy agency. Int J Radiat Oncol Biol Phys 2005;61:145-53. [PubMed]
- van der Zee J, González González D, van Rhoon GC, et al. Comparison of radiotherapy alone with radiotherapy plus hyperthermia in locally advanced pelvic tumours: a prospective, randomised, multicentre trial. Dutch Deep Hyperthermia Group. Lancet 2000;355:1119-25. [PubMed]
- Franckena M, Stalpers LJ, Koper PC, et al. Long-term improvement in treatment outcome after radiotherapy and hyperthermia in locoregionally advanced cervix cancer: an update of the Dutch Deep Hyperthermia Trial. Int J Radiat Oncol Biol Phys 2008;70:1176-82. [PubMed]
- Lutgens L, van der Zee J, Pijls-Johannesma M, et al. Combined use of hyperthermia and radiation therapy for treating locally advanced cervix carcinoma. Cochrane Database Syst Rev 2010.CD006377. [PubMed]
- Wootton JH, Prakash P, Hsu IC, et al. Implant strategies for endocervical and interstitial ultrasound hyperthermia adjunct to HDR brachytherapy for the treatment of cervical cancer. Phys Med Biol 2011;56:3967-84. [PubMed]
- Kim Y, Seol DR, Mohapatra S, et al. Locally targeted delivery of a micron-size radiation therapy source using temperature-sensitive hydrogel. Int J Radiat Oncol Biol Phys 2014;88:1142-7. [PubMed]
- Hodge LS, Downs LS Jr, Chura JC, et al. Localized delivery of chemotherapy to the cervix for radiosensitization. Gynecol Oncol 2012;127:121-5. [PubMed]
- Kong T, Zeng J, Wang X, et al. Enhancement of radiation cytotoxicity in breast-cancer cells by localized attachment of gold nanoparticles. Small 2008;4:1537-43. [PubMed]
- Cho SH. Estimation of tumour dose enhancement due to gold nanoparticles during typical radiation treatments: a preliminary Monte Carlo study. Phys Med Biol 2005;50:N163-73.
- Pan Y, Leifert A, Ruau D, et al. Gold nanoparticles of diameter 1.4 nm trigger necrosis by oxidative stress and mitochondrial damage. Small 2009;5:2067-76. [PubMed]
- Kang B, Mackey MA, El-Sayed MA. Nuclear targeting of gold nanoparticles in cancer cells induces DNA damage, causing cytokinesis arrest and apoptosis. J Am Chem Soc 2010;132:1517-9. [PubMed]
- Jain S, Coulter JA, Hounsell AR, et al. Cell-specific radiosensitization by gold nanoparticles at megavoltage radiation energies. Int J Radiat Oncol Biol Phys 2011;79:531-9. [PubMed]
- Libutti SK, Paciotti GF, Byrnes AA, et al. Phase I and pharmacokinetic studies of CYT-6091, a novel PEGylated colloidal gold-rhTNF nanomedicine. Clin Cancer Res 2010;16:6139-49. [PubMed]
- van Lonkhuijzen L, Thomas G. Palliative radiotherapy for cervical carcinoma, a systematic review. Radiother Oncol 2011;98:287-91. [PubMed]
- Kim DH, Lee JH, Ki YK, et al. Short-course palliative radiotherapy for uterine cervical cancer. Radiat Oncol J 2013;31:216-21. [PubMed]
- Spanos WJ Jr, Wasserman T, Meoz R, et al. Palliation of advanced pelvic malignant disease with large fraction pelvic radiation and misonidazole: final report of RTOG phase I/II study. Int J Radiat Oncol Biol Phys 1987;13:1479-82. [PubMed]
- Lagrange JL, Bondiau PY, Tessier E, et al. Tumoral platinum concentrations in patients treated with repeated low-dose cisplatin as a radiosensitizer. Int J Cancer 1996;68:452-6. [PubMed]


