β(1-3)(1-6)-D-glucans modulate immune status in pigs: potential importance for efficiency of commercial farming
Introduction
Immune system is a system of the biological structures, pathways and mechanisms of mutually interactive systems of an organism that help to protect it against diseases and infections. Initially, β(1-3)(1-6)-D-glucans (term β-glucan will be used throughout the paper) have drawn so much attention worldwide due to their immunomodulatory capabilities in the innate immune system. Numerous types of β-glucans have been isolated from fungi (yeasts, molds and mushrooms), grain and seaweed. They have been extensively studied for their immunological and pharmacological effects and currently more than 4,000 papers describing the mostly immunological activities of β-glucans exist in the literature (1).
The innate immune system is fairly well conserved among vertebrates due to its ancient roots in evolutionary history. It is estimated that the innate system goes as far back as early metazoan—over a billion years ago (2,3). This retention through time of the innate system explains the effects of β-glucans throughout the animal species. Although β-glucan was initially considered to be stimulator of the cellular immunity, in the last decades researchers demonstrated that its biological effects are pleiotropic. On one hand, they are able to absorb a wide range of mycotoxins (4), on the other hand, they stimulate both branches of immunity. In addition, β-glucans were shown to reduce serum cholesterol in hypercholesterolemic animals (5), suppress cancer growth (6), lower stress (7), and regulate blood sugar levels (8). β-Glucan has routinely been used in animal farming; in fish (9), shrimp (10) and in pig feed (11-13). The addition of β-glucan to pig feed altered immune and intestinal functions (12), increased weight (14), reduced peak net glucose flux (15), decreased Th17-related cytokine production (16) and protected against infection (17). In chicken, β-glucan addition was found to reduce the severity of Eimeria infection (18), to upregulate the phagocytosis of macrophages after bacterial infection (19) and to improve growth performance (20).
In this paper, we decided to evaluate the effects of adding β-glucan into commercial feed of piglets. We measured weight, changes in phagocytosis and levels of interleukin 2 (IL-2), cortisol and tumor necrosis factor alpha (TNF-α) in blood. Phagocytosis and IL-2 levels are two of the most often used immunological responses tested in glucan studies, LPS challenge is particularly important in farmed animals.
The objective of this study was to evaluate the effects of this β-glucan on biological characteristics important for health of these farm animals.
Materials and methods
Animals
At the onset of the study, the animals were examined for signs of any disease and all were considered healthy on the basis of a lack of clinically relevant abnormalities. The animals were then randomly assigned to individual groups. None of the used animals developed any disease including lethargy, fever or gastrointestinal problems during this study. Similarly, none of these animals either died or were euthanized during this study. The use of animals was approved by the University of Louisville Institutional Animal Care And Use Committee (IACUC) (#12029). Piglets (all white Yorkshire-Landrace) were purchased from Oak Hill Genetics (Ewing, IL, USA). All animals were grown in conventional conditions.
Materials
Lipopolysaccharides (LPS) from Escherichia coli, and sodium citrate were obtained from Sigma (St. Louis, MO, USA).
Glucan
Insoluble β-glucan is 68.5% pure and isolated from Sacharomyces cerevisiae (Biorigin, Brazil).
Feed
For all experiments, we used Laboratory Porcine Diet Grower #5084 (Purina Mills, Inc., Richmond, IN). The composition of the diet is given in Table 1. After weaning, piglets were placed in individual pens. Weekly body weight was recorded and treatment dosages adjusted accordingly. Piglet feed was supplemented with 15 mg of β-glucan/kg/day.
Phagocytosis
The technique employing phagocytosis of synthetic polymeric microspheres was described earlier (21,22). Briefly: 0.1 mL of peripheral blood (with sodium citrate 1:9) or 0.1 mL of peritoneal fluid was incubated in vitro with 0.05 mL of 2-hydroxyethyl methacrylate particles (HEMA particles; 5×108/mL). The test tubes were incubated at 37 °C for 60 min, with intermittent shaking. Smears were stained with Wright stain. The cells with three or more HEMA particles were considered positive. All experiments were performed in triplicates. At least 300 cells in 60 high power fields were examined in each experiment.
IL-2
Levels of IL-2 were evaluated in serum (1 mL of blood was collected) at the end of experiment using a commercial IL-2 enzyme-linked immunosorbent assay (ELISA) kit as recommended by the manufacturer (R&D Systems, Minneapolis, MN, USA) for pigs.
Evaluation of TNF-α
Levels of TNF-α were evaluated in serum (1 mL of blood was collected) at 1, 2 and 3 hr. after injection of LPS from 14 days after beginning of feedings with β-glucan. The level of TNF-α, Rockford, IL, USA).
Evaluation of cortisol
Levels of cortisol were evaluated in serum (1 mL of blood was collected) at 1, 2 and 3 hr. after injection of LPS from 14 days after beginning of feedings with β-glucan. The levels of cortisol were measured by a commercial ELISA kit (Abnova, Walnut, MA, USA).
Weight
Piglets were fed with the diet (Table 1) with or without β-glucan for 28 days. Weights were determined weekly to calculate average daily gain (ADG) and average daily feed intake (ADFI); data not shown.
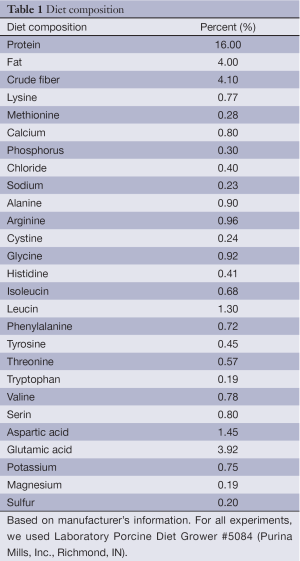
Full table
Statistics
Data were analyzed by Analysis of Variance (ANOVA) using the General liner model (GLM) procedure appropriate for a factorial arrangement of treatments in a randomized block design. The statistical model included the effects of challenge (LPS) and/or dietary supplements. The pen was used as the experimental unit for analysis. A level of P≤0.05 was used to indicate statistically significant results.
Results
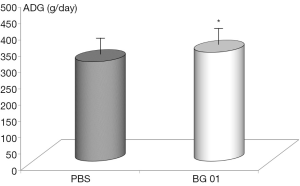
Body weights were recorded every week for the entire duration of the experiment. ADGs were calculated for each of the 7-day periods. The ADG was influenced by the addition of β-glucan. Addition of glucan caused significant increase in piglet model (Figure 1). Average daily food intake did not increase (data not shown).
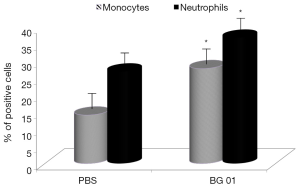
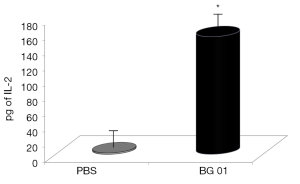
β-Glucans are considered non-specific stimulators of cellular immunity, particularly macrophages. Phagocytosis is therefore one of the main defense reactions, where a stimulating effects of β-glucan can be observed. For our experiments, we used phagocytosis of synthetic microspheres based on 2-hydroxyethyl methacrylate with minimal false positivity. Our results showed that addition of β-glucan significantly potentiated phagocytic activity of both peripheral blood monocytes and neutrophils (Figure 2). Experiments evaluating the effects of glucan on IL-2 production in blood showed that feeding with β-glucan significantly increased the level of IL-2 (Figure 3).
The next two experiments measured the effects of β-glucan supplementation of the body response after a LPS challenge. First, we tested the levels of the stress hormone cortisol. In piglets, glucan significantly abolished the effects of stress (Figure 4). Basal levels of cortisol were 112 nmol/L.
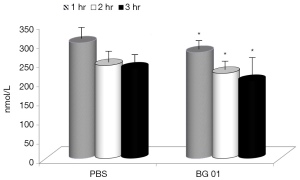
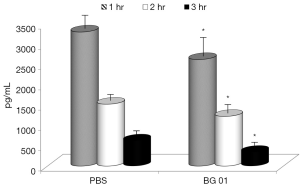
In the last part of our study, we measured concentrations of TNF-α our experimental model, addition of the β-glucan to the feed resulted in significant reduction of TNF-α levels in all three tested intervals (Figure 5). Basal levels of TNF-α were zero.
Discussion
The present study evaluates the effects of insoluble β-glucan on the various reactions in pigs. We found higher ADG when given β-glucan, which is in agreement with previous findings (23-25). The exact mechanisms for these effects are not clear, once the energetic contribution of the β-glucans to the feed at dosages used is neglectable. Plausible explanations might be stress reduction, effects on gastrointestinal tract microbiota (26), on gut permeability (12) and reduction of the negative effects of LPS on feed intake.
With respect of immune system of farm animals, it must be considered that these animals are naturally challenged by LPS during their production period. Even in simpler events, as subclinical diseases or high fiber feeding, higher levels of LPS are produced. LPS will stimulates proinflammatory response, higher TNF-induced cell monolayer disruption and higher tight junction permeability to the intestinal epithelium, leading to higher leakage of LPS to the blood and the initiation of a vicious cycle of proinflammatory response (27). It has a potential to impair feed intake and immune status resulting in the reduction of the production efficiency (28).
β-glucan is well known to be a significant stimulator of immune reaction, particularly of the cellular branch. These effects were repeatedly observed regardless the route of administration (29). For evaluation of the potential effects of our glucan on phagocytosis, we used synthetic polymer microparticles based on hydroxymethacrylate, known for their low negative charge and therefore minimal subjective error during evaluation (22). Our results showed significant stimulation of phagocytosis by both peripheral blood monocytes and neutrophils, which is in agreement with previous finding in mice (29).
The second part of our study focused on effects of β-glucan after an endotoxin challenge. We found significantly lower levels of both cortisol and TNF-α. These results are in agreement with data found in similar model of LPS challenge after weaning (23). The glucan effects found in our study were even stronger than those reported by (24), which might be caused by use of different β-glucans.
In conclusion, β-glucans had significant immunomodulating effects in piglet, strongly suggesting that supplementation of feed with this glucan results in improvement of biological and immunological conditions of farmed animals. In addition, with current efforts to ban on antibiotic and growth promoters, the use of β-glucans as feed additive offers two benefits—natural growth stimulation and natural protection (13).
Acknowledgements
Disclosure: The authors declare no conflict of interest.
References
- Novak M, Vetvicka V. Beta-glucans, history, and the present: immunomodulatory aspects and mechanisms of action. J Immunotoxicol 2008;5:47-57. [PubMed]
- Magnadóttir B. Innate immunity of fish (overview). Fish Shellfish Immunol 2006;20:137-51. [PubMed]
- Du Pasquier L. The immune system of invertebrates and vertebrates. Comp Biochem Physiol B Biochem Mol Biol 2001;129:1-15. [PubMed]
- Yiannikouris A, André G, Poughon L, et al. Chemical and conformational study of the interactions involved in mycotoxin complexation with beta-D-glucans. Biomacromolecules 2006;7:1147-55. [PubMed]
- Fadel JG, Newman RK, Newman CW, et al. Hypocholesterolemic effects of beta-glucans in different barley diets fed to broiler chicks. Nutr Rep Int 1987;35:1049-58.
- Borchers AT, Stern JS, Hackman RM, et al. Mushrooms, tumors, and immunity. Proc Soc Exp Biol Med 1999;221:281-93. [PubMed]
- Vetvicka V, Vancikova Z. Anti-stress action of several orally-given β-glucans. Biomed Pap Med Fac Univ Palacky Olomouc Czech Repub 2010;154:235-8. [PubMed]
- Dong J, Cai F, Shen R, et al. Hypoglycaemic effects and inhibitory effect on intestinal disaccharidases of oat beta-glucan in streptozotocin-induced diabetic mice. Food Chemistry 2011;129:1066-71.
- Welker TL, Lim C, Yildirim-Aksoy M, et al. Immune response and resistance to stress and edwardsiella ictaluri challenge in channel catfish, ictalurus punctatus, fed diets containing commercial whole-cell yeast or yeast subcomponents. J World Aquacult Soc 2007;38:24-35.
- Wang YC, Chang CF, Chen HY. The role of glucans in protection of shrimp against disease. In: Vetvicka V, Novak M. eds. Biology and Chemistry of Beta Glucan. Vol. 2. Bentham Science Publishers, 2013:173-94.
- Hester SN, Comstock SS, Thorum SC, et al. Intestinal and systemic immune development and response to vaccination are unaffected by dietary (1,3/1,6)-β-D-glucan supplementation in neonatal piglets. Clin Vaccine Immunol 2012;19:1499-508. [PubMed]
- Ewaschuk JB, Johnson IR, Madsen KL, et al. Barley-derived β-glucans increases gut permeability, ex vivo epithelial cell binding to E. coli, and naive T-cell proportions in weanling pigs. J Anim Sci 2012;90:2652-62. [PubMed]
- Kogan G, Kocher A. Role of yeast cell wall polysaccharides in pig nutrition and health protection. Livestock Science 2007;109:161-5.
- Dritz SS, Shi J, Kielian TL, et al. Influence of dietary beta-glucan on growth performance, nonspecific immunity, and resistance to Streptococcus suis infection in weanling pigs. J Anim Sci 1995;73:3341-50. [PubMed]
- Hooda S, Matte JJ, Vasanthan T, et al. Dietary oat beta-glucan reduces peak net glucose flux and insulin production and modulates plasma incretin in portal-vein catheterized grower pigs. J Nutr 2010;140:1564-9. [PubMed]
- Ryan MT, O’Shea CJ, Collins CB, et al. Effects of dietary supplementation with Laminaria hyperborea, Laminaria digitata, and Saccharomyces cerevisiae on the IL-17 pathway in the porcine colon. J Anim Sci 2012;90 Suppl 4:263-5. [PubMed]
- Stuyven E, Cox E, Vancaeneghem S, et al. Effect of beta-glucans on an ETEC infection in piglets. Vet Immunol Immunopathol 2009;128:60-6. [PubMed]
- Cox CM, Sumners LH, Kim S, et al. Immune responses to dietary beta-glucan in broiler chicks during an Eimeria challenge. Poult Sci 2010;89:2597-607. [PubMed]
- Chen KL, Weng BC, Chang MT, et al. Direct enhancement of the phagocytic and bactericidal capability of abdominal macrophage of chicks by beta-1,3-1,6-glucan. Poult Sci 2008;87:2242-9. [PubMed]
- Cho JH, Zhang ZF, Kim IH. Effects of single or combined dietary supplementation of β-glucan and kefir on growth performance, blood characteristics and meat quality in broilers. Br Poult Sci 2013;54:216-21. [PubMed]
- Vĕtvicka V, Fornůsek L, Kopecek J, et al. Phagocytosis of human blood leukocytes: a simple micromethod. Immunol Lett 1982;5:97-100. [PubMed]
- Vĕtvicka V, Holub M, Kovárů H, et al. Alpha-fetoprotein and phagocytosis in athymic nude mice. Immunol Lett 1988;19:95-8. [PubMed]
- Eicher SD, McKee CA, Carroll JA, et al. Supplemental vitamin C and yeast cell wall beta-glucan as growth enhancers in newborn pigs and as immunomodulators after an endotoxin challenge after weaning. J Anim Sci 2006;84:2352-60. [PubMed]
- Li J, Li DF, Xing JJ, et al. Effects of beta-glucan extracted from Saccharomyces cerevisiae on growth performance, and immunological and somatotropic responses of pigs challenged with Escherichia coli lipopolysaccharide. J Anim Sci 2006;84:2374-81. [PubMed]
- Hiss S, Sauerwein H. Influence of dietary ss-glucan on growth performance, lymphocyte proliferation, specific immune response and haptoglobin plasma concentrations in pigs. J Anim Physiol Anim Nutr (Berl) 2003;87:2-11. [PubMed]
- Murphy P, Bello FD, O’Doherty JV, et al. Effects of cereal β-glucans and enzyme inclusion on the porcine gastrointestinal tract microbiota. Anaerobe 2012;18:557-65. [PubMed]
- Lambert GP. Stress-induced gastrointestinal barrier dysfunction and its inflammatory effects. J Anim Sci 2009;87:E101-8. [PubMed]
- Jiang ZY, Sun LH, Lin YC, et al. Effects of dietary glycyl-glutamine on growth performance, small intestinal integrity, and immune responses of weaning piglets challenged with lipopolysaccharide. J Anim Sci 2009;87:4050-6. [PubMed]
- Vetvicka V, Vetvickova J. A comparison of injected and orally administered β-glucans. J Am Nutraceut Assoc 2008;11:42-9.


