How to access photodynamic therapy for bile duct carcinoma
Introduction
Surgical resection is a potential curative treatment modality for bile duct carcinomas (BDC); however, complete curability is often difficult because BDC spreads extensively along the bile duct (BD) beyond the lesions observed during preoperative diagnosis (1). Positive surgical margins at the stump of the hepatic duct are a significantly poor prognostic feature in BDC patients who underwent surgical resections (2). To date, there is no evidence supporting the survival advantage of adjuvant radiotherapy and/or chemotherapy after surgery (3,4). On the other hand, in non-resectable advanced BDC, the placement of a metallic stent improved quality of life, although this treatment itself does not contribute a survival benefit (4). However, stent occlusion by tumor ingrowth or stagnant bile frequently occurs. In case of biliary occlusion by tumor growth, local ablation therapies using laser or microwaves have been used for recanalization (1,5). To resolve these problems in BDC treatment, more effective local treatment is necessary.
Photodynamic therapy (PDT), which is a form of laser treatment, has led to remarkable regression of various malignant tumors including BDC since the 1980s (6). PDT is based on the use of laser light to activate photosensitizers and induce specific cytotoxicity in cancer cells. PDT has become a technically feasible and useful modality for the treatment of non-resectable BDC via the percutaneously transhepatic or endoscopic route (7,8). In randomized controlled trials, PDT provided longer survival than BD stenting alone (7,8). A cause for the improved survival is the powerful direct or anti-tumor immunological response induced by PDT. Our group has also reported the benefits of PDT using porfimer sodium and talaporfin sodium in BDC as chemotherapy for non-resectable BDC or adjuvant chemotherapy after surgery for local control (9,10). The most recent articles on PDT for unresectable BDC indicated improvement in liver function, patient quality of life, and prolongation of survival periods with few complications (5,11,12). Thus, PDT also appears to be a promising modality for local treatment in BDC.
To achieve PDT effectiveness of tumor necrosis or apoptosis, conditions of laser radiation are necessary. In BDC, the intraductal space of the target lesion in the BD was limited compared with the digestive tracts, and th the path through which the laser fiber is introduced should be determined. Endoscopy guided laser radiation is necessary, and approaches using one of three endoscopy routes are considered: (I) the percutaneous transhepatic biliary (PTB) route; (II) the percutaneous transjejunal route after surgery; and (III) the endoscopic retrograde biliary (ERB) route via the duodenal papilla. Of these PDT approaches, only Rumalla et al. reported a route of the endoscopic retrograde cholangiography (ERC) (13). Our previous reports described PDT results using these approaches (9). Thus, to our knowledge, there is little information on the clinical applications of PDT approaches in patients with resectable or non-resectable BDC. Based on our experiences, we evaluated the three above routes of PDT in 25 BDC patients in a study that was conducted with the permission of the Institutional Review Board (IRB) in Nagasaki University Hospital.
Patients and methods
Patient
The subjects were 25 patients with BDC who were admitted to the Division of Surgical Oncology, Department of Surgery, Nagasaki University Graduate School of Biomedical Sciences (NUGSBS) between January 2001 and October 2013. The mean age of the patients at the time of surgery was 71 years (range, 53-85 years), and they consisted of 18 males and seven females. Eighteen patients (72%) underwent PDT after surgical resection as an additional local treatment, and seven patients (28%) had unresectable BDC and underwent PDT to release biliary stenosis caused by advanced tumor progression. All patients had good performance status before PDT. BDC was assessed by computed tomography, cholangiography, magnetic resonance imaging, brushing cytology or biopsy via endoscopy before surgery or PDT. In 18 patients undergoing surgical resections prior to PDT, the surgical procedures included left or right hemihepatectomy with resection of the total caudate lobe and the extrahepatic BD in 12 patients, or pancreaticoduodenectomy in six patients. Resections were performed en bloc based on the preoperative imaging diagnosis, and lymph node dissection was performed on the hepatoduodenal ligament, surrounding pancreas head and para-aortic lesions. All hepatic tumors were resected without macroscopic exposure of the amputated section; however, microscopic infiltration of cancer cells was diagnosed by pathological examination during and after surgery. Complete resection failed, requiring additional resection of the hepatic duct in all patients. We used the clinicopathological findings and tumor staging according to the Classification of Biliary Tract Carcinoma in Japan (14).
Our criteria for PDT included BDC without distant metastases or far advanced node metastases based on imaging or pathological diagnosis. PDT was the first-line local treatment in the patients with histological remnant cancer at the amputed BD who underwent surgical resection. Additional chemotherapy and radiation therapy was performed after PDT. The study design was approved by the Ethics Committee of NUGSBS (IRB permission number was #21591777) in 2001, and no conflict of interests was indicated. Signed consent was obtained from each patient before PDT. As PDT for BDC is not been covered by the national health care scheme in Japan, the scientific expense for special research programs in NUGSBS was used for medical expenses associated with PDT in all patients.
The basic research for PDT in BDC in vivo or in vitro was supported by grants-in-aid for scientific research from the Ministry of Education, Science, Sports and Culture of Japan (#21591777 in 2009 and #25462120 in 2013). There is no disclaimer, and the authors declare no conflict of interest.
PDT protocol
All patients who received PDT were in stable condition based on physical examination and laboratory tests after surgery or biliary drainage. During the period of the present series, two photosensitizers were used as the porfimer sodium: (Photofrin; Wyeth Pharmaceuticals, Collegeville, PA, USA, and Wyeth, Tokyo, Japan), a hematoporphyrin derivative in 12 patients (48%) between 2001 and 2008 (15), and talaporfin sodium (NPe6; Laserphyrin® 100 mg for injection; Meiji Seika Pharma., Co Ltd., Tokyo, Japan) in 13 patients (52%) (16). Porfimer sodium was intravenously injected at a dose of 2 mg/kg body weight, 48 hours prior to laser irradiation using the PDT EDL-1 (Hamamatsu Photonics, Hamamatsu, Japan) apparatus (12). An eximer dye pulsed laser with a wavelength of 630 µm (4 millijoules/pulse, 40 Hz) was used. Talaporfin sodium was intravenously injected at a dose of 40 mg/m2 4-6 hours prior to laser treatment and the laser apparatus used was a PDT semiconductor laser with a wavelength 664±2 nm (Panasonic Healthcare Co., Ltd., Tokyo, Japan) (17). After the injection of photosensitizers, the patient stayed in a dark room shielded by a thick black curtain to prevent skin phototoxicity. The light intensity was limited to less than 300 luces for four weeks in the porfimer sodium cases and less than 500 luces for two weeks in the talaporfin sodium cases (17). Protoporphyrin, uroporphyrin (marker of porphyrin metabolites) and conventional blood parameters were monitored before the administration of TPS and 1, 3, 7, 14 and 28 days after PDT.
Prior to PDT in patients who underwent surgical resection, a 16-18 Fr dilated plastic tube was placed via the trans-hepatic or trans-intestinal route to facilitate endoscopy (Figure 1A,B). In only one case (case 5), endoscopy was inserted through a T-shaped tube into the common hepatic duct. In patients with non-resectable BDC with percutaneously transhepatic biliary drainage (PTBD), a 16-18 Fr dilated plastic tube was also placed via the PTBD route to facilitate endoscopy. In patients with non-resectable BDC without PTBD, a 9 Fr preload catheter (ERBD pusher tube, Cook® Inc., Bloomington, USA) (Figure 1C) was first placed in the BD, and the laser fiber was placed near the stenotic lesion via preload catheter. Laser irradiation was applied to two or three target lesions through an endoscope to the anastomotic site of the hepaticojejunostomy (HJ) or the occluding tumor lesion for ten min (Figure 1D). The mean amount of work was 50-100 joules per cm2 of surface by PDT using either porfimer sodium or talaporfin sodium (17). Antibiotic prophylaxis was administered for one day after cholangioscopy.

Statistical analysis
Values for continuous variables were expressed as the means ± SD. For the univariate analysis, differences between the variables were analyzed using Student’s t-test. A two-tailed P value of less than 0.05 was considered significant. Statistical analyses were performed with Statistical Package for the Social Sciences (SPSS) version 18.0 software (SPSS, Chicago, USA).
Results
Demographics
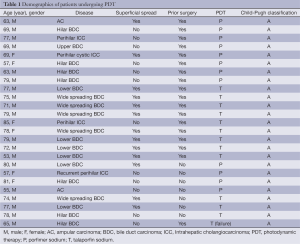
All 25 patients were diagnosed with perihilar intrahepatic cholangiocarcinoma (ICC) (n=4), extrahepatic BDC (n=19), or ampular carcinoma (AC) (n=2) (Table 1). Wide-spread BDC from the hilar to lower BDs was observed in five patients. Perihilar ICC spread to the common BD and case 19 showed local recurrence at the BD stump after right hepatectomy two years ago. Case 21 underwent pancreaticoduodenectomy after two sessions of PDT because the AC could not been cured by PDT. Superficial tumor spreading along the biliary epithelium over 3 cm was observed in 13 patients including lower BDC in six patients and AC in two. One patient had two BDC skip lesions (case 23). PDT using porfimer sodium was performed in eight patients undergoing surgical resection and in four patients without resection. Talaporfin sodium was used in nine patients undergoing surgical resections and in four patients without resection. All patients had a good liver function based on Child-Pugh A classification.
Full table
Tumor findings and treatment status
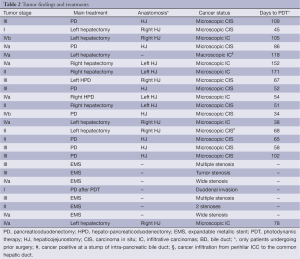
As shown in Table 2, Japanese TNM stage was I in two patients, II in five, III in eight and IVa and IVb in ten. Of the patients undergoing surgical resections with HJ, hepatectomy was performed in nine patients, pancreaticoduodenectomy was performed in eight, and hepatopancreatectomy (HPD) was performed in two. Case 5 underwent left hepatectomy without resection of the caudate lobe and extrahepatic BD. HJ was performed in 16 patients who underwent surgical resections. Remnant BDC after surgical resection was confirmed as a microscopic carcinoma in the epithelium of the BD in 16 patients and as a macroscopically observed tumor infiltration in case 5. In unresectable BDC patients, biliary tumor obstruction was observed in six patients, and the AC invading duodenal wall in case 21. In patients who underwent surgical resections, the mean period from operation for PDT was 87±42 days (range, 24-171 days).
Full table
Route of laser irradiation and results after PDT
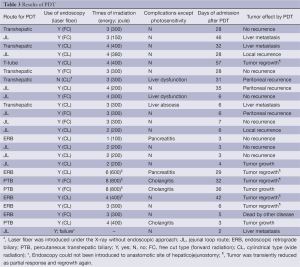
In 18 patients undergoing prior surgical resections, the transhepatic route was selected in five patients (28%), the jejunal loop route in (JL) 11 (61%), the T-tube route in case 5 and the ERC route via papilla vater in case 14 (Table 3). In seven patients with unresectable BDC, the ERC route was selected in four patients (57%), and the PTB route was selected in three (43%). Of the 18 patients who underwent PDT after surgery, one patient (case 25) could not undergo laser irradiation via the JL route because of technical failure of introducing the endoscope to the target region due to a twisted jejunal loop. In case 7, endoscopy guided laser irradiation was not performed. The free cut type of laser fiber (for a forward irradiation) was applied in nine patients, and the cylindrical fiber (for a wide irradiation) was applied in 15. Due to tumor extension, PDT was performed for between one and eight sessions and PDT for two days was performed in four patients. Photodermatitis due to the photosensitizer was observed in ten patients (nine patients by porfimer sodium and one by talaporfin sodium, respectively). Except for the complication of photosensitivity, liver dysfunction was observed in three patients, cholangitis in three and pancreatitis in two. No endoscopy related complications were observed in any patients. The mean period of hospital stay after PDT was 20±17 days (range, 3-57 days). Among them, patients undergoing PDT using porfimer sodium had a significantly longer wait period compared to patients administered talaporfin sodium (36±10 vs. 5±1 days, respectively) (P<0.01). In 16 patients with microscopic BDC after surgery, two patients showed local tumor recurrence, six patients had distant metastases, and seven had no tumor recurrence. In case 5 with macroscopic BDC, tumor was once reduced in size but regrowth was observed at six months. In unresectable BDC, four patients showed a transient tumor reduction but regrowth occurred later. Three patients had no reduction of tumor. Case 23 died due to non-cancer related disease.
Full table
Discussion
The novel treatments such as chemotherapy, brachytherapy or thermal ablation have not showed any evidence of efficacy for local control of tumor progression except surgical curative resections in BDC. However, even surgical resections could not achieve satisfactory control of tumor recurrence or long-term survival, particularly in R1 and R2 resections at this stage (1-4). Matull et al. recently reported that the palliative PDT resulted in survival similar to those with curatively intended R1/R2 resections in biliary tract carcinomas (18). Our result also showed that the completely curative operation only showed prolonged survival (19). Thus only R0 resection (complete curability) provides better survival; however, R0 resection is often difficult due to a cancer positive BD stump based on characteristics of wide spreading along the BD epithelium (20). Wiedmann et al. were the first group that succeeded in applying PDT to resectable BDC as an adjuvant therapy for positive surgical margins after resection (21). Local control with PDT was effective (19) and non-curative operations due to the pathologically positive BD stump remarkably influenced patient survival (18). Our previous result regarding the efficacy of PDT using porfimer sodium tended to show longer cancer-free survival in compared with that in patients without PDT (15 vs. 8 months; P=0.085) (9). In the recent study, 16 of 18 patients who underwent prior surgical resections were local recurrence-free at the irradiated PDT site although distant metastases were observed. Tamada showed that results of metallic stents only for non-resectable BDC were disappointing because of the high rate of occlusion by tumor growth, and therefore, PDT presumably also leads to improved patient quality of life and increased survival in such patients (1). In some randomized controlled trials, PDT provided better performance status, improvement of obstructive jaundice and a longer survival in Europe (6-8,22,23). Thus, PDT is noted as a promising treatment modality to augment conventional anticancer chemotherapy and brachytherapy (3,24). Despite the efficacy of tumor-necrosis by PDT, long periods of skin photosensitivity occur with PDT (25,26).
To achieve adequate laser irradiation to targeted lesions, a fully worked plan is necessary. To maintain the adequate distant between laser fiber and the lesion, endoscopy-guided laser irradiation is thought to be the best approach. During irradiation, it is necessary to check the tip of the laser fiber via a filter for laser light and to avoid contact of the fiber with the surrounding tissues. Furthermore, the dense bile juice might interrupt laser permeability and, therefore, it is necessary to continuously wash with saline. On the other hand, it is necessary to create a 16-18 Fr size access route for approach with the 5-mm-diameter endoscope, and it takes time to dilate the fistula in patients who have undergone prior surgical resections. As shown in the present series, two routes were created: the transhepatic route and the transjejunal route. Most patients underwent hepatectomy or PD with biliary anastomosis. At the begging of the present study, the transhepatic route was applied via a biliary drainage tube. Using this approach, the tube was placed between the intercostal space in the left hepatectomy cases, and dilation of this space was sometimes difficult due to the narrow space. In the right hepatectomy cases, fixation of the route was difficult, and intra-abdominal bile leaks were observed. Therefore, transjejunal approach was recently selected. The jejunal loop was elevated to the BD stump for anastomosis using the retrocolic route, and this loop was often twisted or elongated. In case 2, it took a lot of time to reach at the anastomotic site due to the multiple bending loop. After this experience, the jejunum between the tube placement through the abdominal wall and the anastomotic site was straightened and the distance was kept within 20 cm during the operation. However, as in case 25, we could not find the anastomotic site and technical failure occurred in one of the 25 cases (4%). In case 5, the T-tube placement route was used, which created a large fistula and made it easy to access the common hepatic duct during the endoscopy. Rumalla et al. described the preload catheter technique called a monorail technique to introduce the laser fiber into the narrow BD space (13). Thus, in case 7, to shorten the waiting time for PDT, an entry hole was not dilated, and the 12 Fr size plastic tube was inserted as a preload catheter and the laser fiber was inserted through this tube. However, under X-ray, positioning of the laser tip in the BD space could not been adequately defined, and we could not evaluate the condition of irradiation. Based on this experience, the endoscopy guide is generally necessary as stated in other reports (13,21-23). In unresectable BDC, the ERB route might be applied instead of the PTB route to avoid peritoneal dissemination of cancer cells in the bile (27). However, access using endoscopy is easy and it is possible to repeatedly perform using the transhepatic route. In our series, two types of laser fiber were used: the free cut and the cylindrical type. Both could be inserted into the 5 mm-diameter endoscope. The free cut fiber was applied to remarkable tumor stenosis, and the cylindrical fiber was used for non-visible microscopic cancer lesions at the HJ by considering the range of laser irradiation (10,28). Some patients had complications involving liver dysfunction or cholangitis, which might be due to transient inflammation caused by the PDT. However, endoscopy-related complications were not observed, and PDT was safely performed in the present series.
Our experimental study showed that the cytocidal effect of PDT using talaporfin sodium was significantly increased compared with that of PDT using porfimer sodium (29), and therefore, we expected greater efficacy of tumor necrosis or apoptosis with this next generation PDT. The phototoxicity of talaporfin sodium is lower than that of porfimer sodium (10,16) and thus, hospital stay after PDT was significantly decreased with talaporfin sodium compared to porfimer sodium. With respect to effectiveness of local control of BDC, we could not evaluate the advantages of talaporfin sodium at this stage. Our recent experimental study showed the synergic effect of PDT combined with novel anticancer drugs and neoadjuvant PDT for extended BDC may be effective at reducing tumor range. For this purpose, ERC-guided PDT is necessary.
In conclusion, PDT was safely performed using the endoscopy-guided approach via the transhepatic or ERC route. By considering the disadvantages of both routes, PDT must be adequately achieved for local control of BDC.
Acknowledgements
Disclosure: The authors declare no conflict of interest.
References
- Tamada K, Sugano K. Diagnosis and non-surgical treatment of bile duct carcinoma: developments in the past decade. J Gastroenterol 2000;35:319-25. [PubMed]
- Nakagohri T, Asano T, Kinoshita H, et al. Aggressive surgical resection for hilar-invasive and peripheral intrahepatic cholangiocarcinoma. World J Surg 2003;27:289-93. [PubMed]
- Khan SA, Davidson BR, Goldin R, et al. Guidelines for the diagnosis and treatment of cholangiocarcinoma: consensus document. Gut 2002;51:VI1-9. [PubMed]
- Bowling TE, Galbraith SM, Hatfield AR, et al. A retrospective comparison of endoscopic stenting alone with stenting and radiotherapy in non-resectable cholangiocarcinoma. Gut 1996;39:852-5. [PubMed]
- Indar AA, Lobo DN, Gilliam AD, et al. Percutaneous biliary metal wall stenting in malignant obstructive jaundice. Eur J Gastroenterol Hepatol 2003;15:915-9. [PubMed]
- Dolmans DE, Fukumura D, Jain RK. Photodynamic therapy for cancer. Nat Rev Cancer 2003;3:380-7. [PubMed]
- Berr F, Wiedmann M, Tannapfel A, et al. Photodynamic therapy for advanced bile duct cancer: evidence for improved palliation and extended survival. Hepatology 2000;31:291-8. [PubMed]
- Witzigmann H, Berr F, Ringel U, et al. Surgical and palliative management and outcome in 184 patients with hilar cholangiocarcinoma: palliative photodynamic therapy plus stenting is comparable to r1/r2 resection. Ann Surg 2006;244:230-9. [PubMed]
- Nanashima A, Yamaguchi H, Shibasaki S, et al. Adjuvant photodynamic therapy for bile duct carcinoma after surgery: a preliminary study. J Gastroenterol 2004;39:1095-101. [PubMed]
- Nanashima A, Abo T, Nonaka T, et al. Photodynamic therapy using talaporfin sodium (Laserphyrin®) for bile duct carcinoma: a preliminary clinical trial. Anticancer Res 2012;32:4931-8. [PubMed]
- Ortner ME, Caca K, Berr F, et al. Successful photodynamic therapy for nonresectable cholangiocarcinoma: a randomized prospective study. Gastroenterology 2003;125:1355-63. [PubMed]
- Zoepf T, Jakobs R, Arnold JC, et al. Palliation of nonresectable bile duct cancer: improved survival after photodynamic therapy. Am J Gastroenterol 2005;100:2426-30. [PubMed]
- Rumalla A, Baron TH, Wang KK, et al. Endoscopic application of photodynamic therapy for cholangiocarcinoma. Gastrointest Endosc 2001;53:500-4. [PubMed]
- Japanese Society of Biliary Surgery (JSBS). Part II Extrahepatic bile duct. In: Nagakawa T. eds. Classification of Biliary tract Carcinoma. First English ed. Tokyo: Kanehara & Co., Ltd., 2001:12-32.
- Pereira SP, Aithal GP, Ragunath K, et al. Safety and long term efficacy of porfimer sodium photodynamic therapy in locally advanced biliary tract carcinoma. Photodiagnosis Photodyn Ther 2012;9:287-92. [PubMed]
- Kasuya K, Shimazu M, Suzuki M, et al. Novel photodynamic therapy against biliary tract carcinoma using mono-L: -aspartyl chlorine e6: basic evaluation for its feasibility and efficacy. J Hepatobiliary Pancreat Sci 2010;17:313-21. [PubMed]
- Yoshida T, Tokashiki R, Ito H, et al. Therapeutic effects of a new photosensitizer for photodynamic therapy of early head and neck cancer in relation to tissue concentration. Auris Nasus Larynx 2008;35:545-51. [PubMed]
- Matull WR, Dhar DK, Ayaru L, et al. R0 but not R1/R2 resection is associated with better survival than palliative photodynamic therapy in biliary tract cancer. Liver Int 2011;31:99-107. [PubMed]
- Nanashima A, Tobinaga S, Abo T, et al. Experience of surgical resection for hilar cholangiocarcinomas at a Japanese single cancer institute. Hepatogastroenterology 2012;59:347-50. [PubMed]
- Nanashima A, Sumida Y, Tobinaga S, et al. Characteristics of bile duct carcinoma with superficial extension in the epithelium. World J Surg 2009;33:1255-8. [PubMed]
- Wiedmann M, Caca K, Berr F, et al. Neoadjuvant photodynamic therapy as a new approach to treating hilar cholangiocarcinoma: a phase II pilot study. Cancer 2003;97:2783-90. [PubMed]
- Allison RR, Zervos E, Sibata CH. Cholangiocarcinoma: an emerging indication for photodynamic therapy. Photodiagnosis Photodyn Ther 2009;6:84-92. [PubMed]
- Choi HJ, Moon JH, Ko BM, et al. Clinical feasibility of direct peroral cholangioscopy-guided photodynamic therapy for inoperable cholangiocarcinoma performed by using an ultra-slim upper endoscope (with videos). Gastrointest Endosc 2011;73:808-13. [PubMed]
- Saito H, Takada T, Miyazaki M, et al. Radiation therapy and photodynamic therapy for biliary tract and ampullary carcinomas. J Hepatobiliary Pancreat Surg 2008;15:63-8. [PubMed]
- Ferrario A, Kessel D, Gomer CJ. Metabolic properties and photosensitizing responsiveness of mono-L-aspartyl chlorin e6 in a mouse tumor model. Cancer Res 1992;52:2890-3. [PubMed]
- Roberts WG, Smith KM, McCullough JL, et al. Skin photosensitivity and photodestruction of several potential photodynamic sensitizers. Photochem Photobiol 1989;49:431-8. [PubMed]
- Sugiyama H, Tsuyuguchi T, Sakai Y, et al. Potential role of peroral cholangioscopy for preoperative diagnosis of cholangiocarcinoma. Surg Laparosc Endosc Percutan Tech 2012;22:532-6. [PubMed]
- Harewood GC, Baron TH, Rumalla A, et al. Pilot study to assess patient outcomes following endoscopic application of photodynamic therapy for advanced cholangiocarcinoma. J Gastroenterol Hepatol 2005;20:415-20. [PubMed]
- Nonaka T, Nanashima A, Nonaka M, et al. Advantages of laserphyrin compared with photofrin in photodynamic therapy for bile duct carcinoma. J Hepatobiliary Pancreat Sci 2011;18:592-600. [PubMed]


