A pooled analysis of stereotactic ablative radiotherapy versus lobectomy for operable stage I non-small cell lung cancer: is failure to recruit patients into randomized trials also an answer to the research question?
Non-small cell lung cancer (NSCLC) is among the most common cancers and biggest health care challenges in large parts of the world. Patients with locally advanced NSCLC comprise a heterogeneous group and many of them have unsatisfactory outcomes despite aggressive multimodal treatment approaches. Those with stage I disease (no distant or lymph node metastases; N0 M0) have the highest chance for cure. In the current classification system T-stage is based on primary tumor size (T1a max. 2.0 cm, T1b 2.1-3.0 cm, T2a 3.1-5.0 cm). Tumors larger than 5 cm are classified as stage II, even in the absence of lymph node metastases. In addition stage I requires that these tumors are surrounded by lung or visceral pleura and do not invade the main bronchus (1). Such tumors rarely cause any clinical symptoms, making early detection at this curable stage both challenging and crucial. Screening of high-risk patients using low-dose thoracic computed tomography (CT) imaging is therefore advocated (2).
The historical gold standard for treatment of stage I NSCLC, surgery with lobectomy and systematic hilar and mediastinal lymph node dissection as the sole curative approach, has recently been challenged by hypofractionated stereotactic ablative radiation therapy (SBRT or SABR), due to several reports describing high local control, low regional failure rates and good disease specific survival. SABR has evolved along the same principles that guided successful implementation of high-precision stereotactic radiotherapy for intracranial targets. Groups from Sweden and Japan, and later different European countries and the United States of America developed slightly different technical and planning approaches (3-6). A common feature was short overall treatment time, which is advantageous from a radiobiological point of view and convenient for patients. With the ability to deliver high biologically effective doses (BED) equivalent to more than 100 Gy in conventional 2-Gy fractions regardless of equipment and technique, local control rates rise considerably above those obtained in historical series, where conventional fractionated radiotherapy was administered over many weeks (7). Consequently, local progression as a major source of treatment failure does not limit survival after SABR. A recent multi-institutional analysis reported 2-year local recurrence (LR) of 4% after SBRT with BED >105 Gy as compared to 15% for <105 Gy (P<0.01) (8). Longer treatment duration (≥11 elapsed days) was associated with a 2-year LR of 14% vs. 4% for ≤10 days (P<0.01). After initial uncertainty about the safety of SABR in central tumors and prescription of relatively low equivalent doses, which resulted in sub-optimal LR, reluctance to treat to high BED has decreased and prospective studies have been designed (9).
When implementing SABR, for example, in two of the authors’ previous departments in Munich and Wuerzburg, Germany, around the year 2000, referring physicians almost exclusively selected very old patients or those with serious comorbidity (10). In other words, most of the irradiated patients were not eligible for surgery and some not even for invasive diagnosis, needed to obtain tissue and histology confirmation. Due to this selection bias, overall survival was not comparable to surgical series. Most patients died from cardiovascular and pulmonary comorbidity (as well as second primary cancers), not from the irradiated NSCLC. After a few years and due to the fact that very few patients relapsed, referral patterns changed towards a healthier population, also including occasional patients who refused surgery. All patients were discussed in multidisciplinary tumor boards.
Although initial studies on SABR were heterogeneous (comprised of both prospective and retrospective series with limited patients numbers, in part without histological confirmation of malignancy), the results prompted several phase II trials and later, population-based studies and propensity-matched analyses, which supported the concept of randomized phase III trials in early stage I NSCLC, comparing SABR to surgical resection in operable patients. The ambitious phase III trials (ACOSOG Z4099, ROSEL, STARS) were closed early because of slow accrual. However, ROSEL (a Dutch trial) and STARS (an international trial) shared similar entry criteria and study design, allowing for a pooled analysis. The latter has recently been reported and provides the best available evidence at this point in time (11).
Both randomized studies intended to compare overall survival of operable stage I NSCLC (T1-2a) treated with SABR or lobectomy. In principle, it makes sense to combine the small databases from both studies, which have insufficient statistical power on their own, in order to provide clinically applicable hints and hypotheses. In perspective, the failure to accrue patients resembles previous attempts to compare surgery and radiotherapy or chemoradiotherapy in other scenarios, such as bladder or prostate cancer. It is obviously not very appealing to patients and referring clinicians if the study arms provide extremely different treatment approaches, compared to for example studies where two different radiotherapy fractionation regimens or two different cytotoxic drug cocktails are tested.
The STARS trial attempted to include 420 patients, assuming 82% 3-year overall survival after surgery and a hazard ratio of less than 1.66. The inferiority limit for the ROSEL study was a hazard ratio of 1.35. In essence, the aim was to demonstrate that SABR would provide outcomes comparable to invasive treatment. After disappointing accrual in the time period 2008-2013 the study groups were left with 58 patients from both trials combined. Having said that combining both datasets makes sense from a statistical point of view (increased statistical power), one has to take a closer look at the details. What are the differences between the patients included in STARS and ROSEL, respectively? How cautiously should we interpret the results? As a matter of fact, stratification criteria differed between the studies. Patients without histological confirmation but fulfilling certain clinical criteria pointing strongly towards NSCLC were eligible for the ROSEL but not the STARS trial. Follow-up intervals were different (quite large time intervals of 6 months for 2 years, then annually in the STARS trial), raising the possibility that some of the STARS patients might have gone with undetected relapse at the time of analysis. The differences in equipment and radiotherapy details are probably less important, because sufficient doses were prescribed in both trials. STARS relied on CyberKnife equipment and implanted fiducial markers for image guidance. ROSEL utilized linear accelerators from multiple vendors. Three to five fractions were administered. It is also known from studies of stereotactic radiosurgery for brain metastases that equipment and methodology are not crucial determinants of outcome as long as the radiation dose is sufficiently high (12).
In both study arms median age was 67 years. Most patients had performance status 0 or 1. Other information is shown in Table 1. Comorbidity was not reported. Median follow-up was 40 months in the SABR group and 35 months in the surgery group. Median survival was not reached for either treatment group. Estimated survival favored the SABR group (95% at 3 years compared to 79% after surgery), P=0.037, hazard ratio 0.14 (95% confidence interval 0.017-1.19). Because of short follow-up relapse frequencies are preliminary. No significant differences emerged. Only one patient developed LR (treated with SABR, salvaged by lobectomy). Regional recurrence was numerically higher in the SABR group (13% vs. 4%) without reaching statistical significance. Toxicity after SABR was in the expected range (max. grade III in 10% of the patients), including dyspnea, cough, fatigue, chest wall pain and one incidence of rib fracture. In the surgery group, one patient died of surgical complications and one developed grade IV dyspnea. Other adverse events included grade III dyspnea, infections, chest pain and others resulting in 44% of the surgical patients suffering from grade 3-4 treatment-related adverse events.
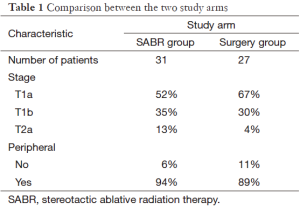
Full table
Since these results suggest that SABR is better tolerated and might lead to better survival, the authors correctly stated that SABR can be considered a treatment option in operable patients needing a lobectomy, and not only a compromise for those the surgeons wont touch. The equipoise suggested by the combined analysis of STARS and ROSEL justifies efforts for additional randomized trials. The latter would be desirable because of the small patient numbers and limited follow-up. In reality it is difficult to expect better recruitment in the future. The present results will definitely not diminish bias and stimulate patients’ interest in surgery. It required tremendous effort to prepare the study protocols and provide the available data from STARS and ROSEL. Unfortunately, recruitment was slow and the conclusions therefore weaker than anticipated. While highly effective local treatment for small stage I NSCLC has become reality, challenges persist regarding control of larger tumors with SABR. Maybe combined modality treatment will contribute to improved outcomes, paralleling the developments in stage III disease. Many patients with lung cancer ultimately die from distant metastases and therefore, better understanding of the processes leading to tumor cell seeding and more effective approaches to control metastatic disease are needed. Preliminary evidence suggests that combinations of systemic and local therapy including SABR should be studied in well-designed, sufficiently powered trials, unless widespread disease precludes reasonable target volumes (13-16).
Acknowledgements
Disclosure: The authors declare no conflict of interest.
References
- Goldstraw P, Crowley J, Chansky K, et al. The IASLC Lung Cancer Staging Project: proposals for the revision of the TNM stage groupings in the forthcoming (seventh) edition of the TNM Classification of malignant tumours. J Thorac Oncol 2007;2:706-14. [PubMed]
- Christensen JD, Chiles C. Low-Dose Computed Tomographic Screening for Lung Cancer. Clin Chest Med 2015;36:147-60. [PubMed]
- Blomgren H, Lax I, Näslund I, et al. Stereotactic high dose fraction radiation therapy of extracranial tumors using an accelerator. Clinical experience of the first thirty-one patients. Acta Oncol 1995;34:861-70. [PubMed]
- Uematsu M, Shioda A, Tahara K, et al. Focal, high dose, and fractionated modified stereotactic radiation therapy for lung carcinoma patients: a preliminary experience. Cancer 1998;82:1062-70. [PubMed]
- Wulf J, Hädinger U, Oppitz U, et al. Stereotactic radiotherapy of targets in the lung and liver. Strahlenther Onkol 2001;177:645-55. [PubMed]
- Timmerman R, Papiez L, Suntharalingam M. Extracranial stereotactic radiation delivery: expansion of technology beyond the brain. Technol Cancer Res Treat 2003;2:153-60. [PubMed]
- Rowell NP, Williams CJ. Radical radiotherapy for stage I/II non-small cell lung cancer in patients not sufficiently fit for or declining surgery (medically inoperable). Cochrane Database Syst Rev 2001;CD002935. [PubMed]
- Kestin L, Grills I, Guckenberger M, et al. Dose-response relationship with clinical outcome for lung stereotactic body radiotherapy (SBRT) delivered via online image guidance. Radiother Oncol 2014;110:499-504. [PubMed]
- Adebahr S, Collette S, Shash E, et al. LungTech, an EORTC Phase II trial of stereotactic body radiotherapy for centrally located lung tumours: a clinical perspective. Br J Radiol 2015;88:20150036. [PubMed]
- Andratschke N, Zimmermann F, Boehm E, et al. Stereotactic radiotherapy of histologically proven inoperable stage I non-small cell lung cancer: patterns of failure. Radiother Oncol 2011;101:245-9. [PubMed]
- Chang JY, Senan S, Paul MA, et al. Stereotactic ablative radiotherapy versus lobectomy for operable stage I non-small-cell lung cancer: a pooled analysis of two randomised trials. Lancet Oncol 2015;16:630-7. [PubMed]
- Nieder C, Grosu AL, Gaspar LE. Stereotactic radiosurgery (SRS) for brain metastases: a systematic review. Radiat Oncol 2014;9:155. [PubMed]
- Nieder C, Tollåli T, Reigstad A, et al. Oligometastatic non-small cell lung cancer: a significant entity outside of specialized cancer centers? Med Princ Pract 2014;23:526-31. [PubMed]
- Ashworth A, Rodrigues G, Boldt G, et al. Is there an oligometastatic state in non-small cell lung cancer? A systematic review of the literature. Lung Cancer 2013;82:197-203. [PubMed]
- Griffioen GH, Toguri D, Dahele M, et al. Radical treatment of synchronous oligometastatic non-small cell lung carcinoma (NSCLC): patient outcomes and prognostic factors. Lung Cancer 2013;82:95-102. [PubMed]
- De Ruysscher D, Wanders R, van Baardwijk A, et al. Radical treatment of non-small-cell lung cancer patients with synchronous oligometastases: long-term results of a prospective phase II trial (Nct01282450). J Thorac Oncol 2012;7:1547-55. [PubMed]


