Improved survival with stereotactic ablative radiotherapy (SABR) over lobectomy for early stage non-small cell lung cancer (NSCLC): addressing the fallout of disruptive randomized data
A recent report by Chang et al. presented a pooled analysis of data from two prospective multicenter randomized trials, the STARS (NCT00840749) and ROSEL (NCT00687986) protocols, comparing lobectomy and stereotactic ablative radiotherapy (SABR) for medically operable patients with T1-2a (<4 cm) N0 M0 non-small cell lung cancer (NSCLC) (1). The results of this analysis were provocative, demonstrating an absolute improvement in overall survival (OS) of 16% at 3 years (95% vs. 79%, P=0.037) and a decrease in grade ≥3 toxicity (10% vs. 48%) in favor of SABR. The primary limitations of this analysis are related to the small patient numbers available from the two trials (58 total patients; 31 treated with SABR and 27 with surgery), which were both closed early following poor accrual. In their discussion, the authors suggest that their results establish a state of equipoise regarding the optimal management for patients with operable early-stage NSCLC (esNSCLC) and should galvanize recruitment to subsequent randomized trials. In the meantime, the foundation of lobectomy as the unassailable gold-standard approach for operable esNSCLC has, for the first time, been shaken by contradictory randomized data.
Reconciliation of the results of the Chang paper with the historic outcomes for esNSCLC requires some familiarity with the literature on SABR for esNSCLC. First, a critical distinction must be made between the prognosis of medically-operable patients in the Chang study and medically-inoperable patients, with the latter representing the majority of patients treated with SABR in reported series to date (2,3). Operable patients are, by definition, those with adequate physiologic reserve to undergo surgery, while inoperable patients are those exceeding acceptable risk thresholds for operative mortality due to factors such as advanced age, pulmonary function, cardiovascular fitness, and performance status (4). Baseline prognostic differences for operable and inoperable patients, irrespective of therapy, are highlighted by a study of 257 esNSCLC patients uniformly treated with SABR, where the 5-year survival rates were 65% vs. 35% (P<0.001) for medically-operable and inoperable patients, respectively (5). These substantial prognostic disparities render survival comparisons between operable patients treated with one modality (e.g., lobectomy) and inoperable patients treated with another modality (e.g., SABR or sublobar resection) inappropriate and confound the majority of retrospective comparisons between surgery and SABR. This point also highlights the significance of the work by Chang and colleagues, as the first prospective comparison of SABR and lobectomy in equivalent populations.
Only recently have studies of SABR specific to medically-operable esNSCLC patients been reported. Recent studies, summarized in Table 1, include the current pooled analysis of multicenter phase III data by Chang et al. (1), the abstract publication of a North American multicenter phase II prospective trial (RTOG 0618) (6), a multicenter Japanese retrospective (8), and a single institution retrospective from the Netherlands (7). In aggregate the reports of SABR for operable esNSCLC demonstrate encouraging 3-year outcomes for OS (80-95%) and progression-free survival (PFS) (60-86%), in addition to estimated 3-year rates of local, regional, and distant failures rates in the range of 4-20%, 10-12%, and 3-20%, respectively. These outcomes compare well with contemporary outcomes for esNSCLC patients managed with surgery alone, where the 3-year OS rates for pT1-2 (<5 cm) N0 tumors are estimated at 70-90% (9). Grade 3 toxicities following SABR for operable esNSCLC have been observed in approximately 5-16% of patients, with no treatment-related deaths. Additional efforts to compare SABR and surgery in equivalent populations have been made using propensity score-matched analyses. In cohorts matched for factors such as age, tumor stage, pulmonary function, comorbidities, and performance status, survival outcomes between SABR and surgery have also appeared equivalent (10-12), with some data suggesting improved oncologic outcomes with SABR (13).
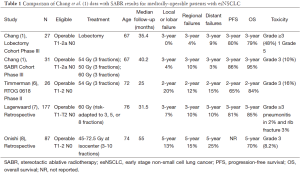
Full table
If one accepts the emerging data from Chang and others suggesting that similar survival outcomes may be achieved with SABR vs. lobectomy for esNSCLC; the next rational question is why? Paradoxically, the analysis by Chang et al. (Table 2) reports a nominally higher, though non-significant, difference in the number of recurrences following SABR (6 events) vs. surgery (3 events), whereas the survival outcomes favored SABR (1 death) over surgery (6 deaths). Inspection of Kaplan-Meier survival curves demonstrates an early separation related to deaths in the surgery group, followed by essentially parallel survival after 18 months.
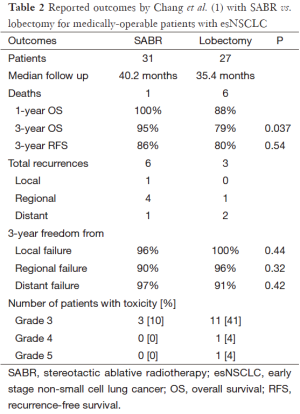
Full table
The phenomenon of early survival curve separation in favor of radiation over surgery has been observed in a number of studies of NSCLC and is most often attributed to differences in treatment-related mortality. In the aforementioned propensity score-matched analyses, population-based data from the Amsterdam Cancer Registry (11) and SEER-Medicare claims data (12) comparing SABR and surgical resection each demonstrate an early OS advantage of SABR and a delayed advantage of surgery, leading to non-significant survival differences overall. Randomized trials in Stage IIIA-N2 NSCLC may also provide useful insights into this trend. An Intergroup trial in the US and Canada included patients receiving induction chemotherapy and 45 Gy of conventionally-fractionated radiation (CFRT) who were randomized to completion of definitive radiation to a total dose of 61 Gy vs. definitive surgical resection (14). An EORTC study randomized patients with response to induction chemotherapy to definitive CFRT or surgery (15). Both of these trials demonstrated an early separation of the survival curves in favor of radiation over surgery, followed by a crossing of the curves between 12 and 36 months, with long-term outcomes non-significantly in favor of surgery. Surgery was associated with an approximate 50% relative reduction in locoregional failures in both trials and statistically significant improvement in PFS in the Intergroup trial.
In each of the studies above, the early separation of the survival curves was attributed to perioperative mortality, while the delayed advantages of surgery were attributed to superior oncologic control; with an overall effect being non-significant differences in OS between groups. Recognizing the apparent oncologic advantage of surgery over definitive CFRT, the authors of the Intergroup trial performed an unplanned subgroup analysis showing a benefit of surgery in patients undergoing lobectomy rather than pneumonectomy, as the latter was associated with greater perioperative mortality (14). The critical difference between these randomized trials and the data presented by Chang (1) is that SABR for esNSCLC is a far better local therapy, associated with local control rates of ≥80-90% in large series (16), compared to CFRT to the lung and mediastinum for Stage IIIA NSCLC, where 50-60% of patients will develop locoregional failures (14,15,17). In light of the early survival advantage afforded to SABR by perioperative mortality, significant survival advantages are likely to be observed in studies where SABR can perform at equivalent, or even near-equivalent, oncologic levels to lobectomy for esNSCLC. We refer to this phenomenon as the “Head Start Effect”.
Operative mortality estimates vary among studies, but are generally reported in the range of 1-4% at 30 days and 2-6% at 90 days following lobectomies for NSCLC (18,19). Baseline mortality risks may be approximately doubled in the setting of pneumonectomy or advanced age, and may be significantly decreased when performed at high-volume centers and in the hands of experienced surgeons (20). Estimates of treatment-related mortality following SABR for esNSCLC have been reported at 0.6% and are frequently absent from contemporary studies following the adoption of conservative dose schedules for centrally located tumors (21,22). In the analysis by Chang (1), it is important to note that only 5 of 27 patients in the surgical cohort underwent a video-assisted thoracoscopic (VATs) lobectomy, and a recent meta-analysis suggests that improved 5-year OS maybe achievable with VATs over open lobectomy (23). Although, at least in this study, even if no patients died due to operative mortality (one) or comorbidities (two) following lobectomy, these differences would have only made the survival outcomes more similar to SABR, but no better.
For future trials of esNSCLC, potential differences in treatment-related mortality beyond conventional time frames of 30 or 90 days may also begin to play a more prominent role in differentiating outcomes between modalities. A report from the National Cancer Database involving 124,418 major pulmonary resections at 1,233 facilities reported ongoing perioperative mortality hazard between 30 and 90 days as an important risk for NSCLC patients undergoing resection (19). However, there is a general paucity of data correlating mortality to operative risk beyond the 90-day mark (24), which may be attributable to the near-absence of randomized data comparing surgical and non-surgical approaches in equivalent esNSCLC populations. Reported rates of surgical complications following lung resections are generally in the range of 30-40% (25). Major surgical complications include arrhythmias, myocardial infarction, respiratory failure, infections, pneumothorax, DVT, and PE, which may be observed in addition to expected decreases in pulmonary function and the promotion of a global pro-inflammatory state (25,26). While most of these complications will not directly lead to mortality during a conventional perioperative period, it is conceivable that they may contribute to a meaningful increase in subacute and delayed mortality hazard in esNSCLC populations with fundamentally limited cardiopulmonary reserve, often presenting with advanced age, heavy smoking histories, and comorbid heart and lung disease.
Until adequately powered randomized trials are completed, reasonable objections to purported equivalence, or potential advantages, of SABR in comparison to lobectomy for esNSCLC will surely remain. Conceptually, it is difficult to accept that SABR, which intends to treat only the tumor with a margin of normal surrounding tissue, could be oncologically equivalent to the removal of a the tumor and tumor-involved lobe of the lung. After all, a landmark randomized trial of lobectomy vs. sublobar resection demonstrated a 3-fold increase in local failures and a strong trend toward inferior OS and cancer-specific survival with sub-lobar therapy (27). If we assume that potential off-target immune enhancement (so-called ‘abscopal effects’) following SABR and potential scattered radiation dose to microscopic disease in hilar or mediastinal lymph nodes do not translate into clinically meaningful benefits, we would submit that SABR and lobectomy are likely not to be equivalent oncologic therapies—at least in terms of local tumor control. Focusing first on the involved lobe, a lobectomy should, in theory, provide 100% in-lobe tumor control. In a hypothetical scenario where SABR provided 100% treated-tumor control, the in-lobe control away from the SABR target will always be less than 100% in an adequately powered study. In illustration of this point, the RTOG 0236 trial of SABR of inoperable esNSCLC reported outstanding 3-year local control rates of 98% at the treated tumor, while in-lobe non-target failures occurred in an additional 7% of patients at 3 years (28) and 13% at the 5-year mark (29). With regards to regional control, hilar and mediastinal nodal dissections (or sampling) in conjunction with lobectomy may potentially reduce regional nodal recurrences in patients undergoing surgery vs. SABR; although, interestingly the 5-year regional failure rates with SABR observed in the range of 7-15% are quite comparable to surgical series (7,29,30). Overall, the window of opportunity for lobectomy to outperform SABR in terms of cancer-specific survival would presumably be found in these potential differences in isolated locoregional failures (that is, without concurrent distant failures) between the two local treatment modalities. However, in the largest available series of SABR for esNSCLC involving 676 patients, isolated locoregional failures were observed in only 6% (42 patients) (13), suggesting a relatively narrow window for surgery to establish superiority in terms of cancer-specific survival. Moreover, it is also reasonable to assume that as more medically-operable patients with esNSCLC are treated with SABR, a greater number of isolated locoregional SABR failures will be surgically-salvageable (31,32). This concept is somewhat analogous to the successful application of salvage mastectomies for recurrent breast cancer after upfront lumpectomy and radiation (33).
There are several additional criticisms of merit regarding the use of SABR for operable esNSCLC, including the frequent use of SABR for patients without a histologic diagnosis and the clinical implications of pathologic upstaging of patients undergoing resection of esNSCLC. In the Chang paper (1), the STARS protocol required histologic confirmation for enrollment, whereas the ROSEL protocol did not, given that the reported likelihood of pathologically benign disease in the setting of radiographic features consistent with malignant disease was estimated to be less than 6% in the Dutch population (34). Notably, the largest analysis on the subject, including 591 patients treated with SABR for histologically-confirmed (209 patients) and clinical-only (382 patients) esNSCLC, demonstrated no differences in survival or local, regional, or distant control between groups (35). Given that a portion of patients will also undergo surgery without preoperative histologic-confirmation when malignancy is strongly suspected (36), non-invasive SABR may carry certain advantages for patients with benign disease in terms of treatment-related morbidity and mortality. On the other hand, surgical resection would ultimately be expected to spare such patients from years of oncologic follow up and anxiety once benign disease is identified (37). For patients with NSCLC, it must also be acknowledged that a wealth of molecular, histologic, and other prognostic information can only be obtained pathologically via either resection or biopsy (36). Finally, despite similar regional recurrence rates following SABR and lobectomy with nodal evaluation (8), nodal upstaging may be observed in as many as 19% of patients following definitive resection and may provide critical information for the guidance of adjuvant therapy (38). Together, these issues underscore the value of pursuing a histologic diagnosis prior to planned SABR delivery, as well as the utility of pre-treatment mediastinal staging procedures in suitable candidates (39), similar to approaches used prior to definitive surgery.
Although small in numbers, the data reported by Chang and colleagues have substantial disruptive implications regarding our time honored approach of surgery-until-proven-otherwise for esNSCLC. In a gathering state of equipoise, as suggested by Chang and others (1,7,8), adequately powered clinical trials comparing lobectomy and SABR for this population are now clearly needed. For thoracic oncologists treating NSCLC, the most common malignancy in men and women combined, it is useful to consider the example set by the landmark trials of breast conservation (40), or we may risk the fate of localized-prostate cancer management—with commendable surgical and radiotherapy options—but no understanding of how they might fare, or what cohorts might benefit most from a given modality, when compared in a well-designed, adequately powered randomized trial (41).
Acknowledgements
Disclosure: The authors declare no conflict of interest.
References
- Chang JY, Senan S, Paul MA, et al. Stereotactic ablative radiotherapy versus lobectomy for operable stage I non-small-cell lung cancer: a pooled analysis of two randomised trials. Lancet Oncol 2015;16:630-7. [PubMed]
- Onishi H, Araki T. Stereotactic body radiation therapy for stage I non-small-cell lung cancer: a historical overview of clinical studies. Jpn J Clin Oncol 2013;43:345-50. [PubMed]
- Soldà F, Lodge M, Ashley S, et al. Stereotactic radiotherapy (SABR) for the treatment of primary non-small cell lung cancer; systematic review and comparison with a surgical cohort. Radiother Oncol 2013;109:1-7. [PubMed]
- British Thoracic Society; Society of Cardiothoracic Surgeons of Great Britain and Ireland Working Party. BTS guidelines: guidelines on the selection of patients with lung cancer for surgery. Thorax 2001;56:89-108. [PubMed]
- Onishi H, Shirato H, Nagata Y, et al. Hypofractionated stereotactic radiotherapy (HypoFXSRT) for stage I non-small cell lung cancer: updated results of 257 patients in a Japanese multi-institutional study. J Thorac Oncol 2007;2:S94-100. [PubMed]
- Timmerman RD, Paulus R, Pass HI, et al. RTOG 0618: Stereotactic body radiation therapy (SBRT) to treat operable early-stage lung cancer patients. J Clin Oncol 2013;31:abstr 7523.
- Lagerwaard FJ, Verstegen NE, Haasbeek CJ, et al. Outcomes of stereotactic ablative radiotherapy in patients with potentially operable stage I non-small cell lung cancer. Int J Radiat Oncol Biol Phys 2012;83:348-53. [PubMed]
- Onishi H, Shirato H, Nagata Y, et al. Stereotactic body radiotherapy (SBRT) for operable stage I non-small-cell lung cancer: can SBRT be comparable to surgery? Int J Radiat Oncol Biol Phys 2011;81:1352-8. [PubMed]
- Rami-Porta R, Ball D, Crowley J, et al. The IASLC Lung Cancer Staging Project: proposals for the revision of the T descriptors in the forthcoming (seventh) edition of the TNM classification for lung cancer. J Thorac Oncol 2007;2:593-602. [PubMed]
- Crabtree TD, Denlinger CE, Meyers BF, et al. Stereotactic body radiation therapy versus surgical resection for stage I non-small cell lung cancer. J Thorac Cardiovasc Surg 2010;140:377-86. [PubMed]
- Palma D, Visser O, Lagerwaard FJ, et al. Treatment of stage I NSCLC in elderly patients: a population-based matched-pair comparison of stereotactic radiotherapy versus surgery. Radiother Oncol 2011;101:240-4. [PubMed]
- Shirvani SM, Jiang J, Chang JY, et al. Lobectomy, sublobar resection, and stereotactic ablative radiotherapy for early-stage non-small cell lung cancers in the elderly. JAMA Surg 2014;149:1244-53. [PubMed]
- Verstegen NE, Oosterhuis JW, Palma DA, et al. Stage I-II non-small-cell lung cancer treated using either stereotactic ablative radiotherapy (SABR) or lobectomy by video-assisted thoracoscopic surgery (VATS): outcomes of a propensity score-matched analysis. Ann Oncol 2013;24:1543-8. [PubMed]
- Albain KS, Swann RS, Rusch VW, et al. Radiotherapy plus chemotherapy with or without surgical resection for stage III non-small-cell lung cancer: a phase III randomised controlled trial. Lancet 2009;374:379-86. [PubMed]
- van Meerbeeck JP, Kramer GW, Van Schil PE, et al. Randomized controlled trial of resection versus radiotherapy after induction chemotherapy in stage IIIA-N2 non-small-cell lung cancer. J Natl Cancer Inst 2007;99:442-50. [PubMed]
- Senthi S, Lagerwaard FJ, Haasbeek CJ, et al. Patterns of disease recurrence after stereotactic ablative radiotherapy for early stage non-small-cell lung cancer: a retrospective analysis. Lancet Oncol 2012;13:802-9. [PubMed]
- Curran WJ, Paulus R, Langer CJ, et al. Sequential vs concurrent chemoradiation for stage III non–small cell lung cancer: randomized Phase III trial RTOG 9410. J Natl Cancer Inst 2011;103:1452-60. [PubMed]
- Bryant AS, Rudemiller K, Cerfolio RJ. The 30-versus 90-day operative mortality after pulmonary resection. Ann Thorac Surg 2010;89:1717-22; discussion 1722-3.
- Pezzi CM, Mallin K, Mendez AS, et al. Ninety-day mortality after resection for lung cancer is nearly double 30-day mortality. J Thorac Cardiovasc Surg 2014;148:2269-77. [PubMed]
- Hannan EL, Radzyner M, Rubin D, et al. The influence of hospital and surgeon volume on in-hospital mortality for colectomy, gastrectomy, and lung lobectomy in patients with cancer. Surgery 2002;131:6-15. [PubMed]
- Grutters JP, Kessels AG, Pijls-Johannesma M, et al. Comparison of the effectiveness of radiotherapy with photons, protons and carbon-ions for non-small cell lung cancer: a meta-analysis. Radiother Oncol 2010;95:32-40. [PubMed]
- Nagata Y, Hiraoka M, Mizowaki T, et al. Survey of stereotactic body radiation therapy in Japan by the Japan 3-D Conformal External Beam Radiotherapy Group. Int J Radiat Oncol Biol Phys 2009;75:343-7. [PubMed]
- Chen FF, Zhang D, Wang YL, et al. Video-assisted thoracoscopic surgery lobectomy versus open lobectomy in patients with clinical stage non-small cell lung cancer: a meta-analysis. Eur J Surg Oncol 2013;39:957-63. [PubMed]
- Discussion. J Thorac Cardiovasc Surg 2014;148:2277-8. [PubMed]
- Allen MS, Darling GE, Pechet TT, et al. Morbidity and mortality of major pulmonary resections in patients with early-stage lung cancer: initial results of the randomized, prospective ACOSOG Z0030 trial. Ann Thorac Surg 2006;81:1013-9; discussion 1019-20. [PubMed]
- Nagahiro I, Andou A, Aoe M, et al. Pulmonary function, postoperative pain, and serum cytokine level after lobectomy: a comparison of VATS and conventional procedure. Ann Thorac Surg 2001;72:362-5. [PubMed]
- Ginsberg RJ, Rubinstein LV. Randomized trial of lobectomy versus limited resection for T1 N0 non-small cell lung cancer. Lung Cancer Study Group. Ann Thorac Surg 1995;60:615-22; discussion 622-3. [PubMed]
- Timmerman R, Paulus R, Galvin J, et al. Stereotactic body radiation therapy for inoperable early stage lung cancer. JAMA 2010;303:1070-6. [PubMed]
- Timmerman RD, Hu C, Michalski J, et al. Long-term Results of RTOG 0236: A Phase II Trial of Stereotactic Body Radiation Therapy (SBRT) in the Treatment of Patients with Medically Inoperable Stage I Non-Small Cell Lung Cancer. Int J Radiat Oncol Biol Phys 2014;90:S30.
- Lou F, Huang J, Sima CS, et al. Patterns of recurrence and second primary lung cancer in early-stage lung cancer survivors followed with routine computed tomography surveillance. J Thorac Cardiovasc Surg 2013;145:75-81; discussion 81-2. [PubMed]
- Bradley J. New territory: surgical salvage for stereotactic body radiation therapy failures in lung cancer. J Thorac Oncol 2010;5:1879-80. [PubMed]
- Chen F, Matsuo Y, Yoshizawa A, et al. Salvage lung resection for non-small cell lung cancer after stereotactic body radiotherapy in initially operable patients. J Thorac Oncol 2010;5:1999-2002. [PubMed]
- Abner AL, Recht A, Eberlein T, et al. Prognosis following salvage mastectomy for recurrence in the breast after conservative surgery and radiation therapy for early-stage breast cancer. J Clin Oncol 1993;11:44-8. [PubMed]
- Verstegen NE, Lagerwaard FJ, Senan S. Developments in early-stage NSCLC: advances in radiotherapy. Ann Oncol 2012;23:x46-51. [PubMed]
- Verstegen NE, Lagerwaard FJ, Haasbeek CJ, et al. Outcomes of stereotactic ablative radiotherapy following a clinical diagnosis of stage I NSCLC: comparison with a contemporaneous cohort with pathologically proven disease. Radiother Oncol 2011;101:250-4. [PubMed]
- Ettinger DS, Wood DE, Akerley W, et al. Non-Small Cell Lung Cancer, Version 6.2015. J Natl Compr Canc Netw 2015;13:515-24. [PubMed]
- Montazeri A, Milroy R, Hole D, et al. Anxiety and depression in patients with lung cancer before and after diagnosis: findings from a population in Glasgow, Scotland. J Epidemiol Community Health 1998;52:203-4. [PubMed]
- Licht PB, Jørgensen OD, Ladegaard L, et al. A national study of nodal upstaging after thoracoscopic versus open lobectomy for clinical stage I lung cancer. Ann Thorac Surg 2013;96:943-9; discussion 949-50. [PubMed]
- Sarwate D, Sarkar S, Krimsky WS, et al. Optimization of mediastinal staging in potential candidates for stereotactic radiosurgery of the chest. J Thorac Cardiovasc Surg 2012;144:81-6. [PubMed]
- Fisher B, Anderson S, Bryant J, et al. Twenty-year follow-up of a randomized trial comparing total mastectomy, lumpectomy, and lumpectomy plus irradiation for the treatment of invasive breast cancer. N Engl J Med 2002;347:1233-41. [PubMed]
- Welz S, Nyazi M, Belka C, et al. Surgery vs. radiotherapy in localized prostate cancer. Which is best? Radiat Oncol 2008;3:23. [PubMed]


