Melanoma: oncogenic drivers and the immune system
Introduction
Melanoma is a malignancy that arises from melanocytes, the pigment producing cells in the body that may be derived from a number of different anatomic sites including skin, mucosal surfaces, conjunctiva, and uveal structures. From the mid-1970s through the mid-2000s, an era that saw the rise and approval of many chemotherapeutic agents and the development of curative regimens for several malignancies, the survival of patients with metastatic melanoma went unchanged (1). In recent years there has been a veritable explosion of progress in understanding melanoma and in exploiting this information for clinical benefit. With the emergence of powerful molecular diagnostic tools, a number of genetic mutations and amplifications or deletions have been identified that appear to drive tumor growth and survival signaling and render these cells sensitive to small-molecule inhibitors. This has led to the development of highly effective signal transduction targeted and immune-targeted therapies. Since 2011, the FDA has approved six agents (ipilimumab, vemurafenib, dabrafenib, trametinib, pembrolizumab, and nivolumab) with four different mechanisms of action [cytotoxic T lymphocyte-associated antigen 4 (CTLA-4) inhibitor, BRAF inhibitors, MEK inhibitor, and PD-1 receptor inhibitor] (2-8). This is a review of the relevant discoveries regarding melanoma biology that have been or are beginning to be translated into transformative therapies.
The story of BRAF in melanoma
Two factors led to the molecular therapy revolution in melanoma. The first was the finding that oncogenic mutations in BRAF are present and drive tumor growth in up to 50% of melanomas (9) with the vast majority of BRAF mutations being found in melanomas that arise from intermittently sun-exposed skin (9,10). The second was the proliferation of small-molecule kinase inhibitors to hit the clinic since 2002 when Philip Cohen predicted that protein kinases would become “the drug targets of the 21st century” (11). BRAF mutations are present in 10% to 20% of melanomas arising in mucosal or acral locations, but 0% of uveal melanomas (UMs) (9,12,13). The most frequent of the BRAF mutations is the T1796A point mutation that results in the substitution of valine (V) for glutamic acid (E) in the second position of codon 600 of exon 15 (V600E), which activates the protein and drives signaling though the MAPK pathway (9). Subsequently, variant mutations have been described involving codon 600, the so-called V600E2, as well as those that result in the substitution of valine with lysine (K) (V600K), aspartic acid (D) (V600D), or arginine (R) (V600R) in relatively small patient cohorts (14). V600K occurs in 20% of BRAF-mutant patients (15) and is associated with advancing age and chronic sun damage (16). The BRAF V600R mutation is the third most prevalent position 600 BRAF mutation and occurs in 5-7% of patients (17).
The BRAF mutations that have been characterized fall into two categories: those that cause RAS-independent activation of MEK and ERK (including BRAF-V600E) and those that have a minimal ability to directly phosphorylate MEK, but instead activate CRAF (RAF1) (18). Depleting BRAF-V600E in vitro decreases ERK activity and induces apoptosis whereas the depletion of BRAF mRNA in melanoma cell lines lacking a BRAF mutation has minimal effect (19). With BRAF-V600E functioning as the dominant oncoprotein in melanoma, depleting CRAF lacks these effects (19-21). In the setting of wild-type BRAF with upstream activation of the RAS pathway, inhibition of BRAF leads to increased CRAF activity and downstream MAPK pathway activity (22-24). This effect involves the enhancement of wild-type RAF oligomerization in the context of upstream pathway activation besides the suppression of monomeric, catalytically active BRAF-V600E by the BRAF inhibitor. This may explain why stably expressed BRAF siRNA in BRAF-V600E human melanoma cells transplanted into immunocompromised mice greatly slowed the growth of these xenografts, but did not completely abrogate tumor growth (21).
Initial efforts to therapeutically target BRAF were focused on small molecules that block a wide range of kinases including RAF (sorafenib, RAF265). Not surprisingly, these agents never were associated with the dramatic effects of the more potent and specific BRAF inhibitors (25,26). Eventually inhibitors that preferentially targeted mutant isoforms of RAF (particularly at the 600 position) (3,14,27) or MEK1/2 (4,28) were tested in the clinic and have revolutionized the treatment of BRAF-mutant melanoma. But despite the successes of single-agent BRAF and MEK inhibitors for the treatment of metastatic, BRAF-mutant melanoma, the reality is that most patients develop disease progression after 6 or 7 months and only a small percentage of patients remain progression-free beyond a year (2,6,13,14).
Mechanisms of resistance to MAPK pathway inhibition can be subdivided according to their temporal occurrence into intrinsic, adaptive and acquired resistance. Intrinsic resistance to BRAF inhibition occurs in almost 50% of all patients with 15% of patients showing no tumor shrinkage at all and 35% of patients achieving only limited tumor reduction. The other 50% of patients initially show tumor shrinkage but subsequently undergo progressive disease with tumor outgrowth because of acquired resistance. Adaptive resistance occurs within hours of drug exposure and reflects the dynamic re-adjustment of signaling pathways at the cellular level (29).
MAPK reactivation in BRAF mutant melanoma
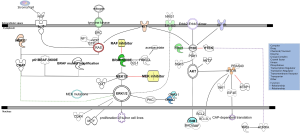
Two major paths to acquired resistance have been recognized: MAPK-dependent and MAPK-independent. MEK/ERK pathway reactivation has been described as the mechanism of resistance to BRAF inhibitors in the majority of cases (30-36). This may be achieved through mechanisms including expression of alternative splicing forms of BRAFV600E (p61 BRAF-V600E), amplification of BRAFV600E, the acquisition of activating mutations in NRAS or MEK (MAP2K1), or loss of NF1. Similarly, COT (MAP3K8) overexpression drives resistance through ERK activation independent of RAF signaling (23,31-33,37-39). Up-regulated receptor tyrosine kinases (RTKs) signal through the SRC-family kinases (SFKs) and lead to pathway reactivation and resistance (Figure 1).
BRAF inhibitors mediate a curious paradox: although they inhibit MEK/ERK signaling in BRAF mutant cells, they activate MEK/ERK signaling in RAS mutant cells. This is because, in the presence of oncogenic RAS, BRAF inhibitors drive the formation of BRAF-CRAF hetero- and homo-dimers containing one partner that is drug bound and one partner that is drug-free. The drug-bound partner drives activation of the drug-free partner through scaffolding or conformational functions, activating CRAF and, consequently, stimulating MEK and ERK hyperactivation (22,24,40). MAPK restoration on the level of RAF can also occur through increased expression and subsequent dimerization of CRAF (41). To overcome both resistance and paradoxical activation of the MEK/ERK pathway, strategies to achieve increased inhibition of the pathway by combined targeting of BRAF and MEK have been tested. The combination of dabrafenib, a BRAF inhibitor, with trametinib, a MEK inhibitor, has been approved by FDA for treating patients with mutant BRAF melanomas (3,42).
Downstream and independent of RAF, expression of the MAPK kinase COT is an additional mechanism of ERK reactivation (31). Further downstream and also independent of ERK1/2, repression of two important apoptotic BH3-only genes, BIM-EL and Bmf, has been observed in the context of resistance (43).
A mutation in MAP2K1/MEK1 (C121S) has been first described by targeted sequencing of a cancer gene panel in a tumor obtained from a patient with melanoma who developed resistance after an initial dramatic response to vemurafenib (44). MEK mutations can be detected in 3-16% of BRAF inhibitor-resistant patients (45,46). Carlino et al. identified patients with both BRAFV600E and MEK1P124 mutant melanoma as a subgroup that is likely to have a poor response to single agent BRAF inhibition but may benefit from combination therapies involving MEK or ERK inhibitors (47) (Figure 1).
NRAS mutations
Mutations upstream of BRAF, in particular activating NRAS mutations occurring at either codon 12 or codon 61 and loss-of-function mutations of NF1, are the MAPK pathway drivers in nearly 30% of melanomas (48-50). Melanoma with NRAS mutations frequently occurs on the trunk (51) or on the upper (52,53) or lower extremities (54).
NRAS and BRAF-V600E mutations are considered to be mutually exclusive and have differential importance in melanoma pathophysiology as both of them are sufficient to constitutively activate the MAPK pathway, whereas NRAS is thought to simultaneously activate the PI3K pathway (55). There is some evidence for the co-occurrence of non-V600E BRAF mutations together with NRAS mutation (22). Indeed, mutations in NRAS were the first oncogenic mutations identified in melanoma, almost 20 years before the discovery of BRAF mutations. It was in 1984 that Albino et al. found a NRAS mutation in 1 of 5 cell lines established from different metastases of a melanoma patient (56). One year later, similar observations were also published by Padua et al. (57).
The NF1 tumor suppressor gene encodes a RAS GTPase- activating protein (RAS GAP) neurofibromin, which negatively regulates RAS by catalyzing the hydrolysis of RAS-GTP to RAS-GDP (58) (Figure 1). NF1 mutations can contribute to melanoma development in at least two genetic settings via distinct mechanisms. One is that in the presence of activating BRAF mutations, NF1 loss prevents BRAF-induced senescence of melanocytes. The second is that in the presence of inactivating BRAF mutations NF1 mutations co-occur with NRAS mutations and that all three RAS (NRAS, KRAS and HRAS) isoforms are required for the tumorigenic properties of these cells (50).
Selective pharmacological inhibition of NRAS remains technically challenging because its GTPase activity has so far precluded the successful design of specific small-molecule antagonists. Also the lower tumor-infiltrating lymphocyte (TIL) grade for melanoma with NRAS mutations suggests it has a more immunosuppressed microenvironment, which may affect its response to immunotherapies (54). Farnesyltransferase inhibitors (FTIs) were hoped to inhibit RAS activation by blocking farnesylation, a key post-translational modification of RAS (59) that is also common in many membrane-localized proteins. The FTI tipifarnib was evaluated in a single-agent, single-arm phase II trial in patients with metastatic melanoma, but the lack of responses among the first 14 patients led to the early closure of the trial (60). The MEK inhibitor binimetinib is associated with a 20% response rate and a median PFS of 4 to 5 months in NRAS-mutant melanoma patients (28). A randomized phase II trial of the MEK inhibitor pimasertib compared to dacarbazine has completed enrollment (NCT01693068) while a randomized phase III trial comparing binimetinib with dacarbazine is underway (NCT01763164).
Even if the phase III trial of binimetinib demonstrates a significant improvement in PFS, the great majority of patients will be in need of next-line therapy within 6 to 8 months. Much less is known about resistance to MEK inhibitors in NRAS-mutant melanoma than resistance to BRAF inhibitors in BRAF-mutant melanoma. In the seminal study of Kwong et al. genes regulating the cell cycle, particularly cyclin-dependent kinase 4 (CDK4) were identified as top candidates for mediating MEK inhibitor resistance in NRAS-mutant melanoma (61). Combining a MEK inhibitor with a CDK4/6 inhibitor was associated with better outcomes than either single agent alone in NRAS xenograft models (61) (Figure 1). The first clinical trial of a MEK-plus-CDK4/6 inhibitor (binimetinib plus LEE011) combination demonstrated tolerability at reasonable doses of both agents, reduction in tumor volume in most patients, and a 33% response rate in NRAS-mutant patients (62). A similar trial evaluating the combination of trametinib plus palbociclib is currently open for patients with all solid tumors (NCT02065063).
The role of PI3K/AKT pathway in melanoma
The MAPK signaling pathway is deregulated in more than 70% of melanomas (NRAS and BRAF mutations) but the PI3K/AKT signaling pathway is also found to be deregulated in more than 50% of these tumors, in part due to genetic mutations and in part due to altered post translational modifications. In addition, the PI3K/AKT pathway, with or without concomitant MAPK reactivation represents an alternative path of resistance to BRAF inhibitors. Increased expression of the insulin-like growth factor 1 receptor (IGF1R) and expression of hepatocyte growth factor (HGF) by stromal cells mediate AKT signaling causing BRAF inhibitor resistance (34,36,63). Loss of PTEN, or deregulation of other genes (e.g., AKT3, BCL2A1, IGF1, and PIB5PA), can also activate the PI3K pathway and cause resistance to BRAF inhibitors (64-68). Approximately 70% of melanomas with mutated BRAF also exhibit inactivation of the tumor suppressor PTEN resulting in constitutive activation of the PI3K signaling pathway (48) (Figure 1). PTEN is functionally lost in the majority of melanomas by mutation, loss of heterozygosity (LOH) and chromosomal loss, methylation-induced transcriptional silencing, or microRNA-dependent mechanisms (69-71). Interestingly, concurrent mutation in BRAF and diminished expression of PTEN are common in melanomas (72,73). For instance, in melanocytic nevi BRAF mutations although initially act mitogen-like manner, eventually, oncogene-induced senescence (OIS) ensues. PTEN depletion abrogates BRAFV600E-induced senescence in human fibroblasts and melanocytes and in murine BRAFV600E-driven nevi depletion of PTEN prompts tumor progression. Therefore the PI3K pathway activation serves as a rate-limiting event in melanomas that arise from nevi, acting by abrogating OIS (74).
The ES cell-expressed Ras (ERAS) proto-oncogene, is a recently discovered member of the Ras family which encodes a protein of 227 amino acids sharing 43%, 46%, and 47% identity with HRAS, NRAS, and KRAS, respectively (75). Overexpression of ERAS causes resistance to BRAF inhibition through hyperactivation of AKT (76) (Figure 1). PI3K/AKT pathway inhibits apoptosis by AKT-mediated phosphorylation of the proapoptotic protein BAD at S136 (77). Serine 112 is another site of BAD phosphorylation regulated by MAPK signaling (78,79), and a full apoptotic response is only achieved when both BADS112 and BADS136 residues are dephosphorylated through the suppression of both AKT and MAPK pathways (80) (Figure 1).
Furthermore HGF signaling induces resistance to BRAF inhibition, because HGF is a known regulator of BAD (81). Perna et al. showed that ERAS-expressing melanoma cells have a higher threshold for the induction of apoptosis and are less sensitive to BRAF inhibitor treatment. ERAS confers resistance to BRAF inhibition by promoting the hyperphosphorylation of BADS112 and BADS136, thus counteracting the inhibition of the MAPK pathway and dephosphorylation of BADS112 alone (76). Combining an AKT inhibitor with a BRAF inhibitor could reverse this phenotype (76). Treatment of cells with the BAD-like BH3 mimetic compound ABT-737 partially rescued the resistance phenotype-induced by HGF, supporting the role of BAD and the Bcl-2 family in this mechanism of resistance (76). Because HGF-dependent resistance operates in patients where stromal expression of HGF can be detected, regulation of the activity of BAD is relevant in determining the response of melanomas to BRAF inhibition. Noting that several components of the Bcl-2 family of proteins have been linked to the response of cells to ABT-263/ABT-737, like MCL-1, BAX, and BIM (82,83), a complex interplay between these proteins and BAD could mediate the overall resistance phenotype in response to HGF.
Three AKT kinase family members, AKT1/protein kinase B (PKB) α, AKT2/PKBβ, and AKT3/PKBγ have been identified in human cells sharing extensive structural similarity (84). However, AKT3 is the predominant isoform active in advanced stage melanomas and AKT3 pathway signaling occurs in almost 70% of melanomas functioning to promote tumorigenesis by deregulating apoptosis (85) (Figure 1). The AKT substrate PRAS40 (proline-rich AKT substrate of 40 kDa) (86) has been identified as an important substrate of the AKT3 kinase, which regulates the apoptotic sensitivity of melanoma cells, thereby promoting melanoma tumorigenesis (87). The majority of melanomas from patients with high levels of phosphorylated (active) AKT also have correspondingly higher levels of phosphorylated PRAS40 (pPRAS40) (85) (Figure 1). PRAS40 is an important downstream target of AKT3 in melanomas, whose phosphorylation leads to pathway signaling that deregulates apoptosis. Targeting PRAS40 or inhibiting upstream AKT3 similarly increased melanoma cell apoptotic sensitivity, causing reduced anchorage-independent growth in culture and delayed tumor development in mice (87).
Phosphoinositide-dependent kinase 1 (PDK1), an immediate downstream effector of PI3K, is a master kinase able to phosphorylate more than 20 members of the AGC kinase family, which includes PKA, AKT, protein kinase C (PKC), p70S6k, and SGK (88). Serum- and glucocorticoid-induced protein kinases 1, 2 and 3 (SGK1, SGK2, and SGK3) are the three isoforms of the SGK family of AGC kinases. SGKs exhibit similar substrate specificity to AKT, and both kinases influence the activity of proteins involved in cell growth, survival, and migration (89). SGKs have been identified as key mediators of PDK1 activity in PTEN wild type melanomas (90). PI3K inhibitor can synergize with PDK1 inhibitors in suppressing BRAFV600E PTEN wild type melanoma growth. PDK1 inhibitor-sensitive melanomas express low levels of pigmentation genes associated with microphthalmia-associated transcription factor (MITF) signaling while PDK1 inhibitor-resistant melanomas express inflammation-related genes, including IL6 and IL3 (90). In addition PDK1-deficient melanomas were found to exhibit reduced ZEB1 and MMP3 expression, pointing to the role of PDK1 in control of the EMT, and explaining the reduced metastasis observed in vivo and attenuated growth in 3D in culture. Inhibition of either PDK1 or SGK3 decreased the phosphorylation of 4EBP1, pointing to possible role of these AGC kinases in the regulation of CAP-dependent translation (Figure 1). Interestingly CAP-dependent translation and specifically eIF4F, another component of this translation initiation complex, was recently linked with resistance to BRAF and MEK inhibitors in BRAF-mutant melanoma, colon, and thyroid cancer cells (91).
Pretreatment overexpression of antiapoptotic BCL2 family members, namely BCL2 and BCL2A1, has also been associated with resistance to BRAF inhibitors and may be an alternative explanation for the limited apoptosis associated with BRAF inhibitors (65,92). In resistant melanoma cell lines, vemurafenib or selumetinib either fail to suppress phosphorylated ERK (P-ERK) or resistance emerges through the activity of mammalian target of rapamycin (mTOR), despite P-ERK suppression and BIM induction (93). This suggests that BIM regulation is MAPK-dependent, but independent of mTOR and BIM up-regulation is not always sufficient to promote apoptosis (93) (Figure 1). Combining vemurafenib with an mTOR or PI3K inhibitor improved cell killing in BRAF-mutant melanomas with ERK-independent resistance to MAPK inhibition (93).
Adaptive resistance
In contrast to acquired resistance, much less is known about the adaptive mechanisms that are rapidly switched on in the presence of RAF inhibitor and promote cell survival. The transduction of signals from activated RTKs has been termed “signalability” (94). This signalability is markedly suppressed in BRAFV600E melanomas, which have high levels of ERK-dependent feedback and markedly decreased sensitivity to extracellular ligands.
BRAF-driven tumors are relatively insensitive to secreted growth factors because of the inability of ligands to induce signaling (95). However, ERK inhibition reduces the ERK-dependent feedback, growth factors can signal, and the antitumor effects of the inhibitor are attenuated. Thus, the signaling network is radically changed and reactivated as an adaptation to inhibition of ERK signaling (94,95). Acquired EGFR and platelet-derived growth factor receptor B (PDGFRB) expression has been found after resistance to BRAF and MEK inhibitors as a result of an adaptive response of the cancer cell population during drug selection (96).
In vitro, MAPK signaling recovers rapidly following BRAF inhibition, in part through the relief of feedback inhibition in the pathway and an increased sensitivity to growth factors such as EGF, neuregulin (NRG-1), HGF and fibroblasts growth factor (FGF) (95). In this context, reactivation of MAPK signaling following BRAF inhibition is important for therapeutic escape with increased levels of cell death and tumor regression being seen when BRAF and MEK are co-targeted (95,97). Initial preclinical models indicate a resetting of the ERK pathway (95) and up-regulation and enhanced NRG1 activation of ERBB3 in BRAFV600 melanoma cells (98). In the presence of NRG1, ERBB3 hetero-dimerizes with ERBB2 to become active (Figure 1). Vemurafenib treatment relieves a negative feedback on ERBB3 transcription, resulting in an increase of ERBB3 at the membrane (Figure 1) (99). Enhanced ERBB3 signals in concert with ERBB2 leading to activation of the PI3K–AKT pathway (36,67,100). Treatment with the anti-ERBB3 monoclonal antibody huHER3-8 out-competes NRG1, prevents the activation of ERBB3 and AKT signaling and enhances the efficacy of vemurafenib treatment (99).
Metabolism and ketones can be a potential Achilles’ heel for BRAF mutant melanoma
Oncogenic BRAF not only controls cell proliferation and survival but also is directly linked to metabolic reprogramming of melanoma cells. Slow-cycling tumor cell populations (such as JARID1B-positive melanoma cells) and tumor cells with an EMT-like phenotype contribute signifıcantly to tumor maintenance and growth and support adaptation and survival during a major stress situation as the sudden interruption of MAPK signaling (101). Different types of cancer, including melanoma, harbor populations of quiescent cells that are drug resistant and whose chromatin states can switch dynamically and reversibly (102). Such quiescent cells are characterized by high expression of the chromatin-remodeling factor JARID1A. The histone 3 lysine 4 demethylase JARID1B/KDM5b, a close homologue of the chromatin-remodeling factor JARID1A, is a marker for a small subpopulation of slow-cycling cells which are essential for continuous tumor growth and repopulation of melanoma (103). In contrast to classic cancer stem cells, this slow-cycling phenotype could be acquired also by cells of the rapidly proliferating tumor bulk. Depending on the oxygen level, a dynamic interconversion between the rapidly proliferating JARID1B-low and the slow-cycling JARID1B-high phenotype has been observed. Slow-cycling JARID1B-high melanoma cells display multi-drug resistance irrespective of the agents used, suggesting a high intrinsic capacity for survival of this subpopulation (103,104). In JARID1B-high cells glycolysis is down-regulated as indicated by low hexokinase expression while a significant activation of the citrate cycle and oxidative phosphorylation occurs. The addition of inhibitors of the mitochondrial electron transport chain (phenformin, rotenone) or ATP synthase (oligomycin A, Bz-423) to vemurafenib significantly improved drug susceptibility and eliminated the intrinsically resistant slow-cycling JARID1B-high subpopulation in vitro and in mouse xenografts (103,104).
A “rewiring” between metabolic and cell signaling pathways was reported by Kang and colleagues. Active BRAF up-regulates its synthetic lethal partner, 3-hydroxy-3-methylglutaryl-CoA lyase (HMGCL), through an octamer transcription factor Oct-1 (105) (Figure 1). Subsequently increased intracellular levels of HMGCL product, acetoacetate (AA), selectively enhance binding of BRAF V600E but not BRAF wild-type to MEK1 in V600E-positive cancer cells and promote activation of MEK-ERK signaling. Therefore this synthetic lethal interaction can be used to good effect in the clinic. Kang et al. showed that biopsy samples from patients with BRAFV600E melanomas have increased levels of HMGCL expression while knockdown of HMGCL compromises the growth of human BRAFV600E-mutant melanomas in immunocompromised mice (105).
Ran signaling in melanoma
The GTPase Ran protein is one of the four key components (CRM1/Exportin 1, RAN/RAN-GTPase, RANGAP1 and RANBP1) of the nucleocytoplasmic transport machinery. In two BRAF mutant melanoma cell lines A375 and 526, Ran, Aurora Kinase A (AurkA) and TERT were found to be significantly overexpressed, while c-myc and PTEN were down-regulated (106). AurkA is one member of a serine/threonine kinase family, which compromises three elements (Aurk A, B and C) that are essential components of the mitotic pathway (107). Overexpression of these kinases has been observed in several tumor types and is associated with advanced clinical stage and poor prognosis (108,109).
Caputo et al. found that Ran knockdown down-regulates AurkA in melanoma cells, suggesting that AurkA protein is a Ran downstream target (106). Additionally AurKA inhibition induced PTEN up-regulation and its action was independent of BRAF mutational status (Figure 1). For instance the SK23Mel BRAF wild type melanoma cell line was also sensitive to treatment with AurkA inhibitor suggesting the role of the Ran-AurkA pathway in melanoma without BRAF mutations. High Ran and AurkA gene expression was found in about 48% and 53%, respectively, of 113 tissue samples from metastatic melanoma patients (106). Therefore there are further possible options for personalized therapy in melanoma alongside treatments targeting BRAF.
KIT alterations in melanoma
Mutations and amplification of KIT are observed in 3% of all melanomas, and are more common in disease arising from mucosal, acral or chronically sun-damaged surfaces. Interestingly, 20% to 30% of melanomas arising in mucosal, acral or chronically sun-damaged skin harbor a mutation or genomic amplification of KIT, a known oncogene with validated inhibitors, which is rarely aberrant in cutaneous melanoma (110). The mutations are in most cases substitution mutations mutually exclusive of BRAF and NRAS mutations and often affect the juxtamembrane or kinase domains of KIT. Activating KIT mutations lead to constitutive activation of KIT tyrosine kinase activity, stimulate the MAPK and PI3K–AKT pathways and may predict response to tyrosine kinase inhibitors such as imatinib, nilotinib, or dasatinib (55,111) (Figure 1). Indeed, there is evidence that a ratio of the presence of KIT mutant to wild-type alleles within a tumor greater than one predicts improved response to targeted therapies (110), which suggests that amplification of wild-type KIT is not predictive of a favorable response. The most common melanoma KIT mutation is L576P, which is found in approximately one-third of cases (112). There are case reports as well as clinical trials reporting the clinical testing of imatinib (110,113-116), dasatinib (117), sorafenib (118), and nilotinib (119). Furthermore, at least in gastrointestinal stromal tumors (GISTs), the acquisition of additional KIT mutations affecting the tyrosine kinase domains in exons 13 and 17, is a common mechanism of resistance to KIT-targeted drugs (120) but this was not the case in the phase II study of nilotinib in melanoma harboring KIT alterations following progression to imatinib (121). Rather, the limited data available suggests that, in melanoma, the development of secondary NRAS mutations (122) and activation of the mTOR pathway by alternative mechanisms may result in secondary resistance (123).
GNAQ and GNA11 mutations in uveal melanoma (UM)
UM, which accounts for about 5% of all melanomas, is a genetically and biologically distinct type of melanoma that arises from choroidal melanocytes (124). More than 80% of UMs contain a mutation in 1 of 2 G-protein subunits, GNAQ and GNA11, rarely seen in cutaneous melanoma (125-127) (Figure 1). GNAQ and GNA11 mutations in melanoma affect codons 209 (approximately 95%) or 183 (5%) and result in complete or partial loss of GTPase activity, respectively, thereby leading to constitutive activation of downstream effector pathways (128,129). Mutant GNAQ and GNA11 directly activate PKC while MAPK pathway activation occurs as a consequence of PKC activation and can be partially or temporarily suppressed by PKC inhibitors, slowing tumor growth activate the MAPK pathway (126,127,130,131) (Figure 1).
Other melanoma gene mutations that offer therapeutic insights include CDNK2A deletions, MITF amplification/alteration resulting in dysregulation of “druggable” antiapoptotic proteins, and PTEN disruption leading to PI3K/AKT activation (132). Increased expression of MITF contributes to melanoma progression and resistance to BRAF pathway inhibition. Müller et al. showed that lack of MITF is associated with more severe resistance to a range of inhibitors, while its presence is required for robust drug responses. MITF levels inversely correlate with the expression of several activated RTKs, most frequently AXL. The MITF-low/AXL-high/drug-resistance phenotype is common among mutant BRAF and NRAS melanoma cell lines. AXL inhibition enhances melanoma cell elimination by BRAF or ERK inhibition (133).
The continuing discovery of recurrently mutated melanoma genes and the lack of identified driver mutations in the subtype without NRAS or BRAF mutation suggest that genetic understanding of this malignancy remains incomplete (134).
The main actors of the immune system in melanoma
Many years ago, the first published evidence, demonstrating the presence of a rich intratumoral lymphocytic infiltrate in cutaneous melanoma, suggested the existence of a host immune response against autologous tumor. Moreover, the authors demonstrated that, when TILs are stimulated with IL-2, they acquire clonal expansion and cytolytic activities against melanoma cells, presenting an innovative method for the generation of lymphocytes with specific antitumor competence, potentially useful in the adoptive immunotherapy of tumors (135). From that time, the key components of the innate and adaptive immunity, with their intricate network of cell-to-cell interactions through the secretion of chemokines, cytokines and growth factors, have progressively been identified and understood both in their physiological and pathological functions (136).
The innate immunity refers to the first nonspecific defense mechanisms, mainly mediated by macrophages, granulocytes and antigen-presenting cells (APC), and induced by the appearance of an unrecognized antigen in the body. Macrophages are a population of cells deriving from circulating monocytes. In melanoma lesions, the low tumor-associated macrophage (TAM) to tumor ratio correlates with cancer aggressiveness and metastasis, whereas the high macrophage to tumor ratio is associated with antitumor response (137). However, the tumor itself can modulate macrophage polarization inducing the production and release of pro-tumor cytokines (macrophage dysfunction) (138). Myeloid-derived suppressor cells (MDSC) increase the expression of pro-inflammatory factors, inhibiting the activity of T and natural killer (NK) cells (139). In patients affected by metastatic melanoma, MDSC population is increased compared with healthy donors and patients with localized disease (140). The dendritic cells (DC) present a crucial role in processing and presenting the antigens. Despite the high proportion of DC within the sentinel lymph nodes of melanoma patients, DCs usually present phenotypic and functional defects that facilitate tumor progression and diffusion. It seems that the degree of DC maturation could affect immune response: mature DCs maintain antitumor activity whereas immature DCs are associated with tolerance (141-143). The NK is a population of innate immune cells mediating apoptosis of the target through the releasing of granules containing perforin and granzymes (144). The relevance of the function of NK cells in cancer immunity has been demonstrated in murine models, where the induction of NK activation via IL-15 could eradicate advanced tumors, independently from the presence of activated T lymphocytes (145). In melanoma lesions, similar to other immune cells, there are an increased number of NK cells that are functionally altered, resulting in a less cytotoxic effect against cancer (146,147).
Unlike innate immune responses, the adaptive immunity is antigen-specific and provides long-lasting protection thanks to the presence of an immunologic memory. Adaptive immune response is sustained by two different classes of white blood cells: B and T lymphocytes (these last including CD4+ and CD8+), mediating the antibody and the cell-mediated immune responses, respectively. A robust lymphocytic infiltration, enriched in CD4+/CD8+ and NK cells, was demonstrated to be strongly associated with improved clinical outcome in patients with cutaneous melanoma (148-151). Moreover, the existence of several melanoma-induced immunomodulation strategies has been widely validated, such as the reduction in peripheral and nodal lymphocytes, mediated by the induction of an imbalance between inhibitory and stimulatory immune factors (152,153).
Despite the fact that CD4+ lymphocytes have long been considered as helper cells, sustaining the activation and proliferation of CD8+ T and B cells, some recent preclinical evidence supports more relevant antitumor activity (154,155). In a melanoma mouse model, the adoptive transfer of tumor-reactive CD4+ lymphocytes into lymphopenic recipients induced T cells expansion and differentiation, leading to the regression of large established tumors (156). However, different types of melanoma-associated antigens (such as Melan-1 and NY-ESO-1) have been related to different prognostic impact of CD4+ T cell responses in late-stage melanoma patients (157,158). In this regard, further studies are currently ongoing to clarify the role and potential therapeutic implications of CD4+ in melanoma. A peculiar subtype of CD4+, called regulatory T-cells (Tregs), plays an essential role in the immune homeostasis by modulating physiological and pathological processes (159). This subtype is characterized by the expression of the transcription factor forkhead box P3 (FOXP3), mediating Tregs’ suppressive functions (160). Much published evidence has demonstrated that functionally immunosuppressive Tregs are overexpressed both in peripheral blood of advanced melanoma patients and in tumor microenvironment of primary tumors, lymph nodes and metastatic lesions (161-164). Although the prognostic role of Tregs infiltration is still under investigation, several retrospective studies reported that the accumulation of Tregs in tumor microenvironment predicts local recurrence and reduced overall survival (165-168). The ratio between functional tumor-infiltrating effector lymphocytes and Tregs in particular seems to represent the most reliable variable correlated with favorable clinical outcome (169). The treatment with IL-2 increased the number of CD4+ FOXP3+ Tregs that remain elevated in progressing patients, but decrease in responding patients, suggesting that the balance between Tregs and effector lymphocytes may impact on clinical outcome and on response to treatment (170).
The tumor infiltrating cytotoxic CD8+ lymphocyte is a key component of the adaptive immune response against melanoma-associated antigens (such as Melan-A and MART-1) and represents a prognostic factor, predicting improved survival in advanced-stage melanoma patients (171). Much evidence is available demonstrating that, whereas the circulating CD8+ cells sustain a strong inflammatory response and cytotoxic activities, those that belong to the tumor microenvironment are functionally tolerant (172,173). The exhaustion profile of CD8+ cells, leading to their inability to proliferate and produce stimulatory cytokines (such as IL-2, IFN and TNF), is mediated by the up-regulation of inhibitory signaling pathways as those induced by CTLA-4 and PD-1 (174).
Similar to what has been observed with the T lymphocytes counterpart, an increased density of tumor-infiltrating B cells, CD19+CD80+ in particular, correlates with a favorable disease outcome in patients with cutaneous melanoma (175,176). Moreover, also for B lymphocytes it has been demonstrated that the disturbed homeostasis of B cells, induced by an unbalanced tumor microenvironment, provides a mechanism supporting the cancer immune escape. In advanced melanoma patients, the B cell population is hyporesponsive to stimulation by CD40 and TLR9 and therefore unable to induce CD4+ T lymphocytes response. The degree of B cell dysfunction correlates with extent of loss of memory CD27+ B cells in peripheral blood (177). Moreover, the existence of pro-tumor B lymphocytes specifically polarized by pro-inflammatory cytokines in order to favor cancer growth and metastasis through the interaction with the adhesion molecule MUC18 has been validated in melanoma patients (178,179).
In addition to the different cell populations, some relevant pathways, closely interacting with the immune system, are recognized as crucial for melanoma development and progression (180). The MAPK signaling pathway has already been described in depth and a further paragraph will explain the rationale supporting the combination strategy with immunotherapy. Several reports demonstrated that the constitutive activation of STAT3 in cancer cells induces an immunosuppressive reaction. STAT3-activated melanoma inhibits multiple suppressive cytokines such as IL-6, IL-10 and VEGF and concurrently induces the expansion and activation of MDSC, Tregs and activated T cells (181). Considering the wide range of functions involving STAT3, the identification of active STAT3 inhibitors may potentially allow to reverse immunosuppression acting on both cancer and immune cells. Several inhibitors targeting STAT3 but also the upstream molecules, such as JAK, are currently under investigation (180). Recently, a melanoma-cell-intrinsic oncogenic pathway contributing to the lack of T cell infiltration has been identified and validated. The expression of WNT/β-catenin pathway has been related to the absence of TILs and to the resistance to anti-PD1 and anti-CTLA-4 monoclonal antibody therapy (182).
Immunomodulatory pathways
The precise regulation of the immunological synapses, a nanoscale connection between immune cells, allows the host to defend against a wide range of pathogens and damaged autologous cells, concurrently attenuating excessive systemic inflammation and preventing the development of autoimmunity processes (183). Unfortunately, these crucial processes provide alternative pathways by which tumor can escape the immune response. To date, several immune receptor-ligand pairs, inducing a stimulatory or an inhibitory signaling, have been identified as potential targets for a pharmacological approach with monoclonal antibodies (184).
Unlike the widely investigated checkpoint inhibitors, to date only preliminary evidence is available for stimulatory immune receptors. CD137 is a member of the tumor necrosis factor (TNF) receptor family, expressed on the cell surface after lymphocyte activation, strongly supporting T cell activation and proliferation, cytokine release and cytolytic activity (185,186). Moreover, preclinical evidence demonstrated that agonistic CD137-specific antibodies may up-regulate CD137 expression on NK cells, enhancing cell degranulation and NK-mediated antibody-dependent cellular toxicity (ADCC) (187). Other members of the same TNF superfamily, such as CD40 and CD27, have been demonstrated to be able to stimulate different types of immune cells (APCs, NK and B cells) in order to enhance the immune response (188,189). Glucocorticoid-induced tumor necrosis factor receptor (GITR) family-related protein is a costimulatory molecule activating proliferation and effector functions of T lymphocytes, concurrently inhibiting Tregs (190). Unlike the previously described receptors, OX40 exerts its stimulatory activity by enhancing T cell proliferation and survival, instead of inducing T cell functional activation (191). Considering the impressive results already obtained and the great expectations deriving from immunotherapy against cancer, several monoclonal antibodies targeting these emerging costimulatory receptors are currently under investigation in early phase clinical trials (184).
To date, the most successful immunotherapy approach, first discovered and validated in melanoma and now widely investigated in several types of tumors, is immune checkpoint inhibition. The CTLA-4 represents the first checkpoint inhibitor to be identified and effectively targeted (192). CTLA-4 is expressed by functionally active T lymphocytes and Tregs and exerts its inhibitory activity through the binding with two ligands (B7-1 or CD80 and B7-2 or CD86) on APC (193) (Figure 2). Preclinical studies on mice demonstrated that the loss of CTLA-4 leads to a massive lymphoproliferation with a rapidly evolving fatal multi-organ tissue destruction (194,195). CTLA-4 down-modulates T cells function through the recruitment of two phosphatases (SHP2 and PPA2), constitutively activating the T cell receptor (TCR)-specific kinases FYN, LCK and ZAP-70, resulting in decreased TCR signaling, and dephosphorylating AKT, a key molecule for T cells activation (able to induce mTOR and NF-κB signaling and IL-2 release). The convincing clinical efficacy observed inhibiting CTLA-4 with specific monoclonal antibodies, such as ipilimumab (7,8) and tremelimumab (196), might be explained through the coexistence of different mechanisms: the unbalanced competitions for ligands between CTLA-4 and its costimulatory antagonist CD28, the decreased sequestration of costimulatory factors and the partial depletion of Tregs in tumor microenvironment (197,198).
The other most investigated checkpoint inhibitor is programmed cell death protein 1 (PD-1), expressed on activated T cells, B cells, NK cells and Tregs (199). The binding of PD-1 with its ligands, PD-L1 (or B7-H1, expressed on several cell types including cancer cells, lymphoid and myeloid cells and APCs) and PD-L2 (or B7-DC, expressed primarily on APCs), leads to T cell exhaustion (200) (Figure 2). PD-1 knockout mice show variable manifestations of autoimmunity, but these disease phenotypes occur later and less prominently than those reported for CTLA-4 knockout animals (201). Structurally, the PD-1 receptor presents tyrosine-based switch motif is able to recruit SHP1 and SHP2 phosphatases. Similar to CTLA-4, also PD-1 inhibits T cell activity in part by decreasing TCR signaling and in part blocking AKT activation, although early experiments demonstrated that these two checkpoint inhibitors do not have fully overlapping functions in immunity (202,203).
Although anti-CTLA-4 and anti-PD-1 antibodies are the most promising agents, other novel checkpoint molecules, for which antibody blockers are already under investigation in preclinical or clinical trials, are currently being studied (204). The lymphocyte activation gene 3 protein (LAG3), a CD4 homolog receptor, is expressed on Tregs, activated T cells, NK cells, B cells and plasmacytoid DC (205,206). The interaction between LAG3 and its ligands, major histocompatibility complex (MHC) class II (207) and the recently discovered galectin-3 (208), through the intracellular KIEELE domain, seems to modulate in different ways T cells function (209) (Figure 2). Unlike CTLA-4 and PD-1, LAG3 knockout mice do not develop autoimmunity diseases (210), however a prominent role of LAG3 emerged supporting the already initiated inflammatory processes through the enhanced of activated T cells and Tregs activity (211).
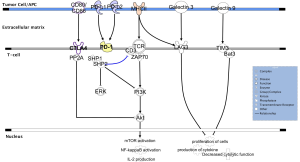
The T cell immunoglobulin and mucin domain-containing 3 (TIM3) is a checkpoint glycoprotein harboring both immunoglobulin and mucin domains, expressed on innate immune cells (such as DCs and monocytes), but also on helper and cytotoxic T lymphocytes (212). When TIM3 binds to galectin-9 (its mostly investigated ligand), the cytoplasmic adaptor protein Bat3 dissociates from the intracellular portion of the receptor, allowing TIM3 to down-regulate the activation and proliferation of lymphocytes (213,214) (Figure 2). Recent evidence demonstrated that TIM3 is usually co-expressed and interacts with CEACAM1 and that this binding is crucial for TIM3 regulatory effects (215). Preclinical evidence suggested that TIM3 and LAG3 may have similar effects in modulating T lymphocytes activity (212).
The NK activity is regulated by a series of complex interconnections among activating receptors, killer inhibitory receptors (KIRs) and ligands, different from a classical MHC I recognition. Interestingly, not all the interactions ligand/KIR are able to induce NK cells activation and KIRs, in this sense, act as immune checkpoint molecules. To date, specific antibodies selectively targeting KIR, therefore preventing their interaction with MHC I molecules and enhancing NK antitumor functions, are currently tested in early clinical trials (216).
The V-domain Ig-containing suppressor of T cell activation (VISTA) is a recently described inhibitor of T cell activation and function, homologous to PD-1 and mainly expressed on myeloid and granulocytes cells (217). A recent analysis showed that treatment with a VISTA monoclonal antibody is able to enhance protective antitumor immunity through multiple mechanisms within the tumor microenvironment (increase in the number and activity of T cells and DC, decrease in the number and function of MDSC and Tregs) (218).
Some non-classical immune checkpoint molecules, not yet fully characterized from a functional point of view, have been identified. B7-H3 is a transmembrane protein, expressed on immune and non-immune cells, able to both up- and down-modulate T cells activation (219,220). T cell ITIM domain (TIGIT) down-regulates the proliferation of T cells, binding to the poliovirus receptor (PVR), that also binds to the T cell surface molecule CD226 (221). IDO (indoleamine 2,3-dioxygenase) pathway induces the catabolism of tryptophan, necessary for T cell survival and function, resulting in an exhausted T cell phenotype and in the immune tolerance (222).
Biological rationale supporting combination therapy in melanoma patients
To date, multiple therapeutic strategies inducing and enhancing antitumor immunity have been developed and are currently employed in the clinic. High-dose IL-2, a type I cytokine activating cytotoxic T cells, was the first immunotherapy approved for metastatic melanoma (223), followed by interferon-α as an adjuvant therapy (224). The anti-CTLA-4 monoclonal antibody ipilimumab was the first treatment able to prolong survival in advanced melanoma patients and was approved in 2011 (7,8). More recently, PD-1 and PD-L1-blocking antibodies demonstrated impressive results in clinical studies with a manageable toxicity profile (225-228). Efforts are now focused on the validation of new agents targeting the immunomodulatory pathways (previously described) and on the implementation of the potential benefit deriving from the checkpoint blockade with a growing interest in combinatorial therapies. There is a strong biological rationale and a wide range of potential clinical applications for synergistic combinations of different immunotherapeutic agents between them, but also of immuno-oncology agents with targeted agents to improve the outcome of patients affected by melanoma (184).
The concomitant inhibition of more than one immunomodulatory pathways may implement the antitumor activity of a single agent because different checkpoints usually play non-redundant roles in the regulation of immunity (229). Impressive results, supporting the rationale of the combination of CTLA-4 and PD-1 antibodies, have been obtained in a randomized trial comparing nivolumab and ipilimumab with ipilimumab monotherapy as a first-line therapy in patients with advanced melanoma. Patients treated with the combination obtained a higher response rate and progression-free survival compared with those receiving ipilimumab monotherapy, without relevant adverse events (230). The rationale supporting the concomitant blockade of different checkpoints, in addition to CTLA-4 and PD-1, derives from the available evidence demonstrating that dysfunctional T cells can commonly co-express multiple checkpoint inhibitors. This data has been extended from the models of chronic infections to those of different of human tumors, such as melanoma and ovarian cancer, where a significant fraction of antigen-specific CD8+ T cells co-express for example LAG3 and PD-1 (231,232). A synergistic immunosuppression mediated by the dual blockade with LAG3 and PD-1 is demonstrated in double-knockout mice, in which most of the implanted tumors were rejected without any evidence of relevant autoimmunity adverse event (204). In addition, TIM3 is nearly universally co-expressed with PD-1 on TILs, inducing an exhausted profile of T cells, unable to proliferate and secrete pro-inflammatory cytokines. TIM3 blocking antibodies potentially useful for a combination strategy are currently under investigation (204). Other early phase trials are currently ongoing with the combination of other checkpoint inhibitor molecules [targeting VISTA (218), KIR (233), B7-H3 (234), TIGIT (221) and IDO (204)] with anti-CTLA-4 and anti-PD-1 monoclonal antibodies. Therefore several early clinical trials are ongoing to investigate the combination of inhibitory and stimulatory agents (184,235).
Another biological rationale strategy in melanoma is represented by the combination of immune checkpoint inhibitors with BRAF or MEK inhibitors. In melanoma, early evidence demonstrated that the driver mutation BRAFV600E could allow an escape to immune response (181). Moreover, BRAF and MEK inhibitors exert, in addition to the direct inhibition of the MAPK pathway, several different concomitant effects on both melanoma and immune cells (236). Much evidence is available demonstrating that targeted therapy increases the expression of melanocyte differentiation antigens (such as MART-1, gp100, TRP-1 and TRP-2) (237) and MHC class I-II (238) on melanoma cells, conferring an enhanced reactivity to antigen-specific lymphocytes. The increased expression of several melanocyte differentiation antigens seems to be related to the release of the transcriptional repression of MITF, induced by the blockade of the MAPK pathway with BRAF inhibitors (239). Regarding immunomodulatory molecules, BRAF inhibition seems to enhance both PD-1 expression on T lymphocytes and PD-L1 expression on melanoma cells, down-regulating the immune response after an initial period of activation (240). This represents a potential mechanism of resistance to BRAF inhibitors, alternative to the MAPK pathway reactivation (241). Moreover, BRAF inhibitors affect the tumor microenvironment reducing the secretion of cytokines (IL-6 and IL-10) and proangiogenic factors (VEGR) and implementing the expression of activated TILs, whose increase during anti-BRAF treatment has been associated with response (237,242). The effect of BRAF inhibitors on BRAF wild-type and non-neoplastic cells, such as T cells, is not yet completely clarified. A recent study demonstrated that RAF inhibitors could enhance T cell activation in a concentration-dependent manner in vitro and in vivo, paradoxically activating ERK signaling via transactivation of RAF dimers. This activation requires the engagement of TCR that activates ERK pathway through RAS (243). Regarding MEK inhibitors, although only preliminary evidence is available, they seem to down-regulate T lymphocyte proliferation, cytokine production, and antigen-specific expansion, without having an impact on CD4+ or CD8+ T cells viability (244). This observation suggests the hypothesis that a theoretical combination between immunotherapy and a MEK inhibitor may potentially have deleterious effect, abrogating the possibility of synergism (245). Nevertheless, recent data demonstrated that the immunomodulatory effects of trametinib on activated T cells are multifaceted and variable according to the context. Both dabrafenib and trametinib alone or in combination are able to increase the proportion of TILs; moreover trametinib increases CD4+ cells when used alone and also CD8+ cells, when administered in combination with an anti-PD-1 antibody (246). Another in vivo study supported the rationale and potential activity of the triple combination therapy of BRAF and MEK inhibitors with immunotherapy in BRAF mutant melanoma (247). Some data are available regarding the potential enhancement of NK and DC activity during BRAF inhibitor treatment (248); whereas the amount of MDSC declines in melanoma patients responding to these targeted agents (140).
Conclusions
Fundamental discoveries over the course of the past three decades have brought a renewed optimism for the treatment of patients with metastatic melanoma: the identification of oncogenic mutations, the elucidation of the molecular signaling resulting from these mutations, and major progress in understanding tumor immunology and immunoescape mechanisms. Clinical results with molecular targeted and immunotherapeutic agents have been so remarkable as to elicit from some the word “cure” in the same sentence as “cancer”.
Despite the impressive results obtained with the introduction of immunotherapy, the progressively deepening understanding of the molecular biology of melanoma might help to identify strategies to overcome resistance, to improve the proportion of patients responding to immunotherapeutic agents, to determine the optimal therapeutic sequence and to validate the synergistic combination approach, making this once incurable disease curable.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Korn EL, Liu PY, Lee SJ, et al. Meta-analysis of phase II cooperative group trials in metastatic stage IV melanoma to determine progression-free and overall survival benchmarks for future phase II trials. J Clin Oncol 2008;26:527-34. [PubMed]
- Chapman PB, Hauschild A, Robert C, et al. Improved survival with vemurafenib in melanoma with BRAF V600E mutation. N Engl J Med 2011;364:2507-16. [PubMed]
- Flaherty KT, Infante JR, Daud A, et al. Combined BRAF and MEK inhibition in melanoma with BRAF V600 mutations. N Engl J Med 2012;367:1694-703. [PubMed]
- Flaherty KT, Robert C, Hersey P, et al. Improved survival with MEK inhibition in BRAF-mutated melanoma. N Engl J Med 2012;367:107-14. [PubMed]
- Hamid O, Robert C, Daud A, et al. Safety and tumor responses with lambrolizumab (anti-PD-1) in melanoma. N Engl J Med 2013;369:134-44. [PubMed]
- Hauschild A, Grob JJ, Demidov LV, et al. Dabrafenib in BRAF-mutated metastatic melanoma: a multicentre, open-label, phase 3 randomised controlled trial. Lancet 2012;380:358-65. [PubMed]
- Hodi FS, O'Day SJ, McDermott DF, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med 2010;363:711-23. [PubMed]
- Robert C, Thomas L, Bondarenko I, et al. Ipilimumab plus dacarbazine for previously untreated metastatic melanoma. N Engl J Med 2011;364:2517-26. [PubMed]
- Davies H, Bignell GR, Cox C, et al. Mutations of the BRAF gene in human cancer. Nature 2002;417:949-54. [PubMed]
- Curtin JA, Fridlyand J, Kageshita T, et al. Distinct sets of genetic alterations in melanoma. N Engl J Med 2005;353:2135-47. [PubMed]
- Cohen P. Protein kinases--the major drug targets of the twenty-first century? Nat Rev Drug Discov 2002;1:309-15. [PubMed]
- Menzies AM, Haydu LE, Visintin L, et al. Distinguishing clinicopathologic features of patients with V600E and V600K BRAF-mutant metastatic melanoma. Clin Cancer Res 2012;18:3242-9. [PubMed]
- Sosman JA, Kim KB, Schuchter L, et al. Survival in BRAF V600-mutant advanced melanoma treated with vemurafenib. N Engl J Med 2012;366:707-14. [PubMed]
- Flaherty KT, Puzanov I, Kim KB, et al. Inhibition of mutated, activated BRAF in metastatic melanoma. N Engl J Med 2010;363:809-19. [PubMed]
- Long GV, Menzies AM, Nagrial AM, et al. Prognostic and clinicopathologic associations of oncogenic BRAF in metastatic melanoma. J Clin Oncol 2011;29:1239-46. [PubMed]
- El-Osta H, Falchook G, Tsimberidou A, et al. BRAF mutations in advanced cancers: clinical characteristics and outcomes. PloS One 2011;6:e25806. [PubMed]
- Lovly CM, Dahlman KB, Fohn LE, et al. Routine multiplex mutational profiling of melanomas enables enrollment in genotype-driven therapeutic trials. PloS One 2012;7:e35309. [PubMed]
- Smalley KS, Xiao M, Villanueva J, et al. CRAF inhibition induces apoptosis in melanoma cells with non-V600E BRAF mutations. Oncogene 2009;28:85-94. [PubMed]
- Hingorani SR, Jacobetz MA, Robertson GP, et al. Suppression of BRAF(V599E) in human melanoma abrogates transformation. Cancer Res 2003;63:5198-202. [PubMed]
- Karasarides M, Chiloeches A, Hayward R, et al. B-RAF is a therapeutic target in melanoma. Oncogene 2004;23:6292-8. [PubMed]
- Sumimoto H, Miyagishi M, Miyoshi H, et al. Inhibition of growth and invasive ability of melanoma by inactivation of mutated BRAF with lentivirus-mediated RNA interference. Oncogene 2004;23:6031-9. [PubMed]
- Heidorn SJ, Milagre C, Whittaker S, et al. Kinase-dead BRAF and oncogenic RAS cooperate to drive tumor progression through CRAF. Cell 2010;140:209-21. [PubMed]
- Poulikakos PI, Persaud Y, Janakiraman M, et al. RAF inhibitor resistance is mediated by dimerization of aberrantly spliced BRAF(V600E). Nature 2011;480:387-90. [PubMed]
- Hatzivassiliou G, Song K, Yen I, et al. RAF inhibitors prime wild-type RAF to activate the MAPK pathway and enhance growth. Nature 2010;464:431-5. [PubMed]
- Flaherty KT, Lee SJ, Zhao F, et al. Phase III trial of carboplatin and paclitaxel with or without sorafenib in metastatic melanoma. J Clin Oncol 2013;31:373-9. [PubMed]
- Hauschild A, Agarwala SS, Trefzer U, et al. Results of a phase III, randomized, placebo-controlled study of sorafenib in combination with carboplatin and paclitaxel as second-line treatment in patients with unresectable stage III or stage IV melanoma. J Clin Oncol 2009;27:2823-30. [PubMed]
- Falchook GS, Long GV, Kurzrock R, et al. Dabrafenib in patients with melanoma, untreated brain metastases, and other solid tumours: a phase 1 dose-escalation trial. Lancet 2012;379:1893-901. [PubMed]
- Ascierto PA, Schadendorf D, Berking C, et al. MEK162 for patients with advanced melanoma harbouring NRAS or Val600 BRAF mutations: a non-randomised, open-label phase 2 study. Lancet Oncol 2013;14:249-56. [PubMed]
- Roesch A. Tumor heterogeneity and plasticity as elusive drivers for resistance to MAPK pathway inhibition in melanoma. Oncogene 2015;34:2951-7. [PubMed]
- Girotti MR, Pedersen M, Sanchez-Laorden B, et al. Inhibiting EGF receptor or SRC family kinase signaling overcomes BRAF inhibitor resistance in melanoma. Cancer Discov 2013;3:158-67. [PubMed]
- Johannessen CM, Boehm JS, Kim SY, et al. COT drives resistance to RAF inhibition through MAP kinase pathway reactivation. Nature 2010;468:968-72. [PubMed]
- Nazarian R, Shi H, Wang Q, et al. Melanomas acquire resistance to B-RAF(V600E) inhibition by RTK or N-RAS upregulation. Nature 2010;468:973-7. [PubMed]
- Shi H, Moriceau G, Kong X, et al. Melanoma whole-exome sequencing identifies (V600E)B-RAF amplification-mediated acquired B-RAF inhibitor resistance. Nat Commun 2012;3:724. [PubMed]
- Straussman R, Morikawa T, Shee K, et al. Tumour micro-environment elicits innate resistance to RAF inhibitors through HGF secretion. Nature 2012;487:500-4. [PubMed]
- Vergani E, Vallacchi V, Frigerio S, et al. Identification of MET and SRC activation in melanoma cell lines showing primary resistance to PLX4032. Neoplasia 2011;13:1132-42. [PubMed]
- Villanueva J, Vultur A, Lee JT, et al. Acquired resistance to BRAF inhibitors mediated by a RAF kinase switch in melanoma can be overcome by cotargeting MEK and IGF-1R/PI3K. Cancer Cell 2010;18:683-95. [PubMed]
- Emery CM, Vijayendran KG, Zipser MC, et al. MEK1 mutations confer resistance to MEK and B-RAF inhibition. Proc Natl Acad Sci U S A 2009;106:20411-6. [PubMed]
- Whittaker SR, Theurillat JP, Van Allen E, et al. A genome-scale RNA interference screen implicates NF1 loss in resistance to RAF inhibition. Cancer Discov 2013;3:350-62. [PubMed]
- Holderfield M, Deuker MM, McCormick F, et al. Targeting RAF kinases for cancer therapy: BRAF-mutated melanoma and beyond. Nat Rev Cancer 2014;14:455-67. [PubMed]
- Poulikakos PI, Zhang C, Bollag G, et al. RAF inhibitors transactivate RAF dimers and ERK signalling in cells with wild-type BRAF. Nature 2010;464:427-30. [PubMed]
- Montagut C, Sharma SV, Shioda T, et al. Elevated CRAF as a potential mechanism of acquired resistance to BRAF inhibition in melanoma. Cancer Res 2008;68:4853-61. [PubMed]
- Long GV, Stroyakovskiy D, Gogas H, et al. Combined BRAF and MEK inhibition versus BRAF inhibition alone in melanoma. N Engl J Med 2014;371:1877-88. [PubMed]
- Shao Y, Aplin AE. BH3-only protein silencing contributes to acquired resistance to PLX4720 in human melanoma. Cell Death Differ 2012;19:2029-39. [PubMed]
- Wagle N, Emery C, Berger MF, et al. Dissecting therapeutic resistance to RAF inhibition in melanoma by tumor genomic profiling. J Clin Oncol 2011;29:3085-96. [PubMed]
- Rizos H, Menzies AM, Pupo GM, et al. BRAF inhibitor resistance mechanisms in metastatic melanoma: spectrum and clinical impact. Clin Cancer Res 2014;20:1965-77. [PubMed]
- Van Allen EM, Wagle N, Sucker A, et al. The genetic landscape of clinical resistance to RAF inhibition in metastatic melanoma. Cancer Discov 2014;4:94-109. [PubMed]
- Carlino MS, Fung C, Shahheydari H, et al. Preexisting MEK1P124 mutations diminish response to BRAF inhibitors in metastatic melanoma patients. Clin Cancer Res 2015;21:98-105. [PubMed]
- Hodis E, Watson IR, Kryukov GV, et al. A landscape of driver mutations in melanoma. Cell 2012;150:251-63. [PubMed]
- Omholt K, Platz A, Kanter L, et al. NRAS and BRAF mutations arise early during melanoma pathogenesis and are preserved throughout tumor progression. Clin Cancer Res 2003;9:6483-8. [PubMed]
- Maertens O, Johnson B, Hollstein P, et al. Elucidating distinct roles for NF1 in melanomagenesis. Cancer Discov 2013;3:338-49. [PubMed]
- Hacker E, Hayward NK, Dumenil T, et al. The association between MC1R genotype and BRAF mutation status in cutaneous melanoma: findings from an Australian population. J Invest Dermatol 2010;130:241-8. [PubMed]
- Ellerhorst JA, Greene VR, Ekmekcioglu S, et al. Clinical correlates of NRAS and BRAF mutations in primary human melanoma. Clin Cancer Res 2011;17:229-35. [PubMed]
- Edlundh-Rose E, Egyhazi S, Omholt K, et al. NRAS and BRAF mutations in melanoma tumours in relation to clinical characteristics: a study based on mutation screening by pyrosequencing. Melanoma Res 2006;16:471-8. [PubMed]
- Thomas NE, Edmiston SN, Alexander A, et al. ASsociation between nras and braf mutational status and melanoma-specific survival among patients with higher-risk primary melanoma. JAMA Oncol 2015;1:359-68. [PubMed]
- Flaherty KT, Hodi FS, Fisher DE. From genes to drugs: targeted strategies for melanoma. Nat Rev Cancer 2012;12:349-61. [PubMed]
- Albino AP, Le Strange R, Oliff AI, et al. Transforming ras genes from human melanoma: a manifestation of tumour heterogeneity? Nature 1984;308:69-72. [PubMed]
- Padua RA, Barrass NC, Currie GA. Activation of N-ras in a human melanoma cell line. Mol Cell Biol 1985;5:582-5. [PubMed]
- Bernards A, Settleman J. GAPs in growth factor signalling. Growth factors 2005;23:143-9. [PubMed]
- Sebti SM. Protein farnesylation: implications for normal physiology, malignant transformation, and cancer therapy. Cancer Cell 2005;7:297-300. [PubMed]
- Gajewski TF, Salama AK, Niedzwiecki D, et al. Phase II study of the farnesyltransferase inhibitor R115777 in advanced melanoma (CALGB 500104). J Transl Med 2012;10:246. [PubMed]
- Kwong LN, Costello JC, Liu H, et al. Oncogenic NRAS signaling differentially regulates survival and proliferation in melanoma. Nat Med 2012;18:1503-10. [PubMed]
- Sosman JA, Kittaneh M, Lolkema MPJK, et al. A phase 1b/2 study of LEE011 in combination with binimetinib (MEK162) in patients with NRAS-mutant melanoma: Early encouraging clinical activity. J Clin Oncol 2014;32:abstr 9009.
- Wilson TR, Fridlyand J, Yan Y, et al. Widespread potential for growth-factor-driven resistance to anticancer kinase inhibitors. Nature 2012;487:505-9. [PubMed]
- Paraiso KH, Xiang Y, Rebecca VW, et al. PTEN loss confers BRAF inhibitor resistance to melanoma cells through the suppression of BIM expression. Cancer Res 2011;71:2750-60. [PubMed]
- Haq R, Yokoyama S, Hawryluk EB, et al. BCL2A1 is a lineage-specific antiapoptotic melanoma oncogene that confers resistance to BRAF inhibition. Proc Natl Acad Sci U S A 2013;110:4321-6. [PubMed]
- Ye Y, Li Q, Hu WL, et al. Loss of PI(4,5)P2 5-Phosphatase A Contributes to Resistance of Human Melanoma Cells to RAF/MEK Inhibitors. Transl Oncol 2013;6:470-81. [PubMed]
- Shao Y, Aplin AE. Akt3-mediated resistance to apoptosis in B-RAF-targeted melanoma cells. Cancer Res 2010;70:6670-81. [PubMed]
- Hilmi C, Larribere L, Giuliano S, et al. IGF1 promotes resistance to apoptosis in melanoma cells through an increased expression of BCL2, BCL-X(L), and survivin. J Invest Dermatol 2008;128:1499-505. [PubMed]
- Guldberg P. Disruption of the MMAC1/PTEN gene by deletion or mutation is a frequent event in malignant melanoma. Cancer Res 1997;57:3660-3. [PubMed]
- Mirmohammadsadegh A, Marini A, Nambiar S, et al. Epigenetic silencing of the PTEN gene in melanoma. Cancer Res 2006;66:6546-52. [PubMed]
- Zhou XP, Gimm O, Hampel H, et al. Epigenetic PTEN silencing in malignant melanomas without PTEN mutation. Am J Pathol 2000;157:1123-8. [PubMed]
- Daniotti M, Oggionni M, Ranzani T, et al. BRAF alterations are associated with complex mutational profiles in malignant melanoma. Oncogene 2004;23:5968-77. [PubMed]
- Lin WM, Baker AC, Beroukhim R, et al. Modeling genomic diversity and tumor dependency in malignant melanoma. Cancer Res 2008;68:664-73. [PubMed]
- Vredeveld LC, Possik PA, Smit MA, et al. Abrogation of BRAFV600E-induced senescence by PI3K pathway activation contributes to melanomagenesis. Genes Dev 2012;26:1055-69. [PubMed]
- Takahashi K, Mitsui K, Yamanaka S. Role of ERas in promoting tumour-like properties in mouse embryonic stem cells. Nature 2003;423:541-5. [PubMed]
- Perna D, Karreth FA, Rust AG, et al. BRAF inhibitor resistance mediated by the AKT pathway in an oncogenic BRAF mouse melanoma model. Proc Natl Acad Sci U S A 2015;112:E536-45. [PubMed]
- Datta SR, Dudek H, Tao X, et al. Akt phosphorylation of BAD couples survival signals to the cell-intrinsic death machinery. Cell 1997;91:231-41. [PubMed]
- Bonni A, Brunet A, West AE, et al. Cell survival promoted by the Ras-MAPK signaling pathway by transcription-dependent and -independent mechanisms. Science 1999;286:1358-62. [PubMed]
- Fang X, Yu S, Eder A, et al. Regulation of BAD phosphorylation at serine 112 by the Ras-mitogen-activated protein kinase pathway. Oncogene 1999;18:6635-40. [PubMed]
- She QB, Solit DB, Ye Q, et al. The BAD protein integrates survival signaling by EGFR/MAPK and PI3K/Akt kinase pathways in PTEN-deficient tumor cells. Cancer Cell 2005;8:287-97. [PubMed]
- Kakazu A, Chandrasekher G, Bazan HE. HGF protects corneal epithelial cells from apoptosis by the PI-3K/Akt-1/Bad- but not the ERK1/2-mediated signaling pathway. Invest Ophthalmol Vis Sci 2004;45:3485-92. [PubMed]
- Cragg MS, Jansen ES, Cook M, et al. Treatment of B-RAF mutant human tumor cells with a MEK inhibitor requires Bim and is enhanced by a BH3 mimetic. J Clin Invest 2008;118:3651-9. [PubMed]
- Sale MJ, Cook SJ. The BH3 mimetic ABT-263 synergizes with the MEK1/2 inhibitor selumetinib/AZD6244 to promote BIM-dependent tumour cell death and inhibit acquired resistance. Biochem J 2013;450:285-94. [PubMed]
- Brazil DP, Yang ZZ, Hemmings BA. Advances in protein kinase B signalling: AKTion on multiple fronts. Trends Biochem Sci 2004;29:233-42. [PubMed]
- Stahl JM, Sharma A, Cheung M, et al. Deregulated Akt3 activity promotes development of malignant melanoma. Cancer Res 2004;64:7002-10. [PubMed]
- Kovacina KS, Park GY, Bae SS, et al. Identification of a proline-rich Akt substrate as a 14-3-3 binding partner. J Biol Chem 2003;278:10189-94. [PubMed]
- Madhunapantula SV, Sharma A, Robertson GP. PRAS40 deregulates apoptosis in malignant melanoma. Cancer Res 2007;67:3626-36. [PubMed]
- Pearce LR, Komander D, Alessi DR. The nuts and bolts of AGC protein kinases. Nat Rev Mol Cell Biol 2010;11:9-22. [PubMed]
- Bruhn MA, Pearson RB, Hannan RD, et al. Second AKT: the rise of SGK in cancer signalling. Growth Factors 2010;28:394-408. [PubMed]
- Scortegagna M, Lau E, Zhang T, et al. PDK1 and SGK3 Contribute to the Growth of BRAF-Mutant Melanomas and Are Potential Therapeutic Targets. Cancer Res 2015;75:1399-412. [PubMed]
- Boussemart L, Malka-Mahieu H, Girault I, et al. eIF4F is a nexus of resistance to anti-BRAF and anti-MEK cancer therapies. Nature 2014;513:105-9. [PubMed]
- Frederick DT, Salas Fragomeni RA, Schalck A, et al. Clinical profiling of BCL-2 family members in the setting of BRAF inhibition offers a rationale for targeting de novo resistance using BH3 mimetics. PloS One 2014;9:e101286. [PubMed]
- Corcoran RB, Rothenberg SM, Hata AN, et al. TORC1 suppression predicts responsiveness to RAF and MEK inhibition in BRAF-mutant melanoma. Sci Transl Med 2013;5:196ra98.
- Rosell R, Karachaliou N, Morales-Espinosa D, et al. Adaptive resistance to targeted therapies in cancer. Transl Lung Cancer Res 2013;2:152-9. [PubMed]
- Lito P, Pratilas CA, Joseph EW, et al. Relief of Profound Feedback Inhibition of Mitogenic Signaling by RAF Inhibitors Attenuates Their Activity in BRAFV600E Melanomas. Cancer Cell 2012;22:668-82. [PubMed]
- Sun C, Wang L, Huang S, et al. Reversible and adaptive resistance to BRAF(V600E) inhibition in melanoma. Nature 2014;508:118-22. [PubMed]
- Paraiso KH, Fedorenko IV, Cantini LP, et al. Recovery of phospho-ERK activity allows melanoma cells to escape from BRAF inhibitor therapy. Br J Cancer 2010;102:1724-30. [PubMed]
- Abel EV, Basile KJ, Kugel CH, et al. Melanoma adapts to RAF/MEK inhibitors through FOXD3-mediated upregulation of ERBB3. J Clin Invest 2013;123:2155-68. [PubMed]
- Kugel CH 3rd, Hartsough EJ, Davies MA, et al. Function-blocking ERBB3 antibody inhibits the adaptive response to RAF inhibitor. Cancer Res 2014;74:4122-32. [PubMed]
- Deng W, Gopal YN, Scott A, et al. Role and therapeutic potential of PI3K-mTOR signaling in de novo resistance to BRAF inhibition. Pigment Cell Melanoma Res 2012;25:248-58. [PubMed]
- Dummer R, Flaherty KT. Resistance patterns with tyrosine kinase inhibitors in melanoma: new insights. Curr Opin Oncol 2012;24:150-4. [PubMed]
- Sharma SV, Lee DY, Li B, et al. A chromatin-mediated reversible drug-tolerant state in cancer cell subpopulations. Cell 2010;141:69-80. [PubMed]
- Roesch A, Vultur A, Bogeski I, et al. Overcoming intrinsic multidrug resistance in melanoma by blocking the mitochondrial respiratory chain of slow-cycling JARID1B(high) cells. Cancer Cell 2013;23:811-25. [PubMed]
- Yuan P, Ito K, Perez-Lorenzo R, et al. Phenformin enhances the therapeutic benefit of BRAF(V600E) inhibition in melanoma. Proc Natl Acad Sci U S A 2013;110:18226-31. [PubMed]
- Kang HB, Fan J, Lin R, et al. Metabolic Rewiring by Oncogenic BRAF V600E Links Ketogenesis Pathway to BRAF-MEK1 Signaling. Mol Cell 2015;59:345-58. [PubMed]
- Caputo E, Wang E, Valentino A, et al. Ran signaling in melanoma: implications for the development of alternative therapeutic strategies. Cancer Lett 2015;357:286-96. [PubMed]
- Vader G, Lens SM. The Aurora kinase family in cell division and cancer. Biochim Biophys Acta 2008;1786:60-72.
- Reiter R, Gais P, Jütting U, et al. Aurora kinase A messenger RNA overexpression is correlated with tumor progression and shortened survival in head and neck squamous cell carcinoma. Clin Cancer Res 2006;12:5136-41. [PubMed]
- Nadler Y, Camp RL, Schwartz C, et al. Expression of Aurora A (but not Aurora B) is predictive of survival in breast cancer. Clin Cancer Res 2008;14:4455-62. [PubMed]
- Carvajal RD, Antonescu CR, Wolchok JD, et al. KIT as a therapeutic target in metastatic melanoma. JAMA 2011;305:2327-34. [PubMed]
- Jiang X, Zhou J, Yuen NK, et al. Imatinib targeting of KIT-mutant oncoprotein in melanoma. Clin Cancer Res 2008;14:7726-32. [PubMed]
- Beadling C, Jacobson-Dunlop E, Hodi FS, et al. KIT gene mutations and copy number in melanoma subtypes. Clin Cancer Res 2008;14:6821-8. [PubMed]
- Guo J, Si L, Kong Y, et al. Phase II, open-label, single-arm trial of imatinib mesylate in patients with metastatic melanoma harboring c-Kit mutation or amplification. J Clin Oncol 2011;29:2904-9. [PubMed]
- Hodi FS, Corless CL, Giobbie-Hurder A, et al. Imatinib for melanomas harboring mutationally activated or amplified KIT arising on mucosal, acral, and chronically sun-damaged skin. J Clin Oncol 2013;31:3182-90. [PubMed]
- Hodi FS, Friedlander P, Corless CL, et al. Major response to imatinib mesylate in KIT-mutated melanoma. J Clin Oncol 2008;26:2046-51. [PubMed]
- Lutzky J, Bauer J, Bastian BC. Dose-dependent, complete response to imatinib of a metastatic mucosal melanoma with a K642E KIT mutation. Pigment Cell Melanoma Res 2008;21:492-3. [PubMed]
- Woodman SE, Trent JC, Stemke-Hale K, et al. Activity of dasatinib against L576P KIT mutant melanoma: molecular, cellular, and clinical correlates. Mol Cancer Ther 2009;8:2079-85. [PubMed]
- Quintás-Cardama A, Lazar AJ, Woodman SE, et al. Complete response of stage IV anal mucosal melanoma expressing KIT Val560Asp to the multikinase inhibitor sorafenib. Nat Clin Pract Oncol 2008;5:737-40. [PubMed]
- Cho JH, Kim KM, Kwon M, Kim JH, Lee J. Nilotinib in patients with metastatic melanoma harboring KIT gene aberration. Invest New Drugs 2012;30:2008-14. [PubMed]
- Antonescu CR. The GIST paradigm: lessons for other kinase-driven cancers. J Pathol 2011;223:251-61. [PubMed]
- Carvajal RD, Lawrence DP, Weber JS, et al. Phase II Study of Nilotinib in Melanoma Harboring KIT Alterations Following Progression to Prior KIT Inhibition. Clin Cancer Res 2015;21:2289-96. [PubMed]
- Minor DR, Kashani-Sabet M, Garrido M, et al. Sunitinib therapy for melanoma patients with KIT mutations. Clin Cancer Res 2012;18:1457-63. [PubMed]
- Si L, Xu X, Kong Y, et al. Major response to everolimus in melanoma with acquired imatinib resistance. J Clin Oncol 2012;30:e37-40. [PubMed]
- Singh AD, Bergman L, Seregard S. Uveal melanoma: epidemiologic aspects. Ophthalmol Clin North Am 2005;18:75-84. viii. [PubMed]
- Curtin JA, Busam K, Pinkel D, et al. Somatic activation of KIT in distinct subtypes of melanoma. J Clin Oncol 2006;24:4340-6. [PubMed]
- Van Raamsdonk CD, Bezrookove V, Green G, et al. Frequent somatic mutations of GNAQ in uveal melanoma and blue naevi. Nature 2009;457:599-602. [PubMed]
- Van Raamsdonk CD, Griewank KG, Crosby MB, et al. Mutations in GNA11 in uveal melanoma. N Engl J Med 2010;363:2191-9. [PubMed]
- Markby DW, Onrust R, Bourne HR. Separate GTP binding and GTPase activating domains of a G alpha subunit. Science 1993;262:1895-901. [PubMed]
- Kalinec G, Nazarali AJ, Hermouet S, et al. Mutated alpha subunit of the Gq protein induces malignant transformation in NIH 3T3 cells. Mol Cell Biol 1992;12:4687-93. [PubMed]
- Hemesath TJ, Price ER, Takemoto C, et al. MAP kinase links the transcription factor Microphthalmia to c-Kit signalling in melanocytes. Nature 1998;391:298-301. [PubMed]
- Chen X, Wu Q, Tan L, et al. Combined PKC and MEK inhibition in uveal melanoma with GNAQ and GNA11 mutations. Oncogene 2014;33:4724-34. [PubMed]
- Chin L, Garraway LA, Fisher DE. Malignant melanoma: genetics and therapeutics in the genomic era. Genes Dev 2006;20:2149-82. [PubMed]
- Müller J, Krijgsman O, Tsoi J, et al. Low MITF/AXL ratio predicts early resistance to multiple targeted drugs in melanoma. Nat Commun 2014;5:5712. [PubMed]
- Menzies AM, Long GV. Systemic treatment for BRAF-mutant melanoma: where do we go next? Lancet Oncol 2014;15:e371-81. [PubMed]
- Muul LM, Spiess PJ, Director EP, et al. Identification of specific cytolytic immune responses against autologous tumor in humans bearing malignant melanoma. J Immunol 1987;138:989-95. [PubMed]
- Gajewski TF, Schreiber H, Fu YX. Innate and adaptive immune cells in the tumor microenvironment. Nat Immunol 2013;14:1014-22. [PubMed]
- Ellyard JI, Quah BJ, Simson L, et al. Alternatively activated macrophage possess antitumor cytotoxicity that is induced by IL-4 and mediated by arginase-1. J Immunother 2010;33:443-52. [PubMed]
- Elgert KD, Alleva DG, Mullins DW. Tumor-induced immune dysfunction: the macrophage connection. J Leukoc Biol 1998;64:275-90. [PubMed]
- Lindau D, Gielen P, Kroesen M, et al. The immunosuppressive tumour network: myeloid-derived suppressor cells, regulatory T cells and natural killer T cells. Immunology 2013;138:105-15. [PubMed]
- Schilling B, Paschen A. Immunological consequences of selective BRAF inhibitors in malignant melanoma: Neutralization of myeloid-derived suppressor cells. Oncoimmunology 2013;2:e25218. [PubMed]
- Gerlini G, Di Gennaro P, Mariotti G, et al. Human Langerhans cells are immature in melanoma sentinel lymph nodes. Blood 2012;119:4807-8. [PubMed]
- Gerlini G, Urso C, Mariotti G, et al. Plasmacytoid dendritic cells represent a major dendritic cell subset in sentinel lymph nodes of melanoma patients and accumulate in metastatic nodes. Clin Immunol 2007;125:184-93. [PubMed]
- Walzer T, Dalod M, Robbins SH, et al. Natural-killer cells and dendritic cells: "l'union fait la force Blood 2005;106:2252-8. [PubMed]
- Marcus A, Gowen BG, Thompson TW, et al. Recognition of tumors by the innate immune system and natural killer cells. Adv Immunol 2014;122:91-128. [PubMed]
- Liu RB, Engels B, Arina A, et al. Densely granulated murine NK cells eradicate large solid tumors. Cancer Res 2012;72:1964-74. [PubMed]
- Balsamo M, Pietra G, Vermi W, et al. Melanoma immunoediting by NK cells. Oncoimmunology 2012;1:1607-9. [PubMed]
- Balsamo M, Scordamaglia F, Pietra G, et al. Melanoma-associated fibroblasts modulate NK cell phenotype and antitumor cytotoxicity. Proc Natl Acad Sci U S A 2009;106:20847-52. [PubMed]
- Al-Batran SE, Rafiyan MR, Atmaca A, et al. Intratumoral T-cell infiltrates and MHC class I expression in patients with stage IV melanoma. Cancer Res 2005;65:3937-41. [PubMed]
- Clark WH Jr, Elder DE, Guerry D 4th, et al. Model predicting survival in stage I melanoma based on tumor progression. J Natl Cancer Inst 1989;81:1893-904. [PubMed]
- Clemente CG, Mihm MC Jr, Bufalino R, et al. Prognostic value of tumor infiltrating lymphocytes in the vertical growth phase of primary cutaneous melanoma. Cancer 1996;77:1303-10. [PubMed]
- McKay K, Moore PC, Smoller BR, et al. Association between natural killer cells and regression in melanocytic lesions. Hum Pathol 2011;42:1960-4. [PubMed]
- Koziner B, Cosimi AB, Bloch KJ. Distribution of latex-ingesting cells, T cells, and B cells in the peripheral blood of patients with malignant melanoma. J Natl Cancer Inst 1975;55:1295-9. [PubMed]
- Mansfield AS, Holtan SG, Grotz TE, et al. Regional immunity in melanoma: immunosuppressive changes precede nodal metastasis. Mod Pathol 2011;24:487-94. [PubMed]
- Muranski P, Boni A, Antony PA, et al. Tumor-specific Th17-polarized cells eradicate large established melanoma. Blood 2008;112:362-73. [PubMed]
- Perez-Diez A, Joncker NT, Choi K, et al. CD4 cells can be more efficient at tumor rejection than CD8 cells. Blood 2007;109:5346-54. [PubMed]
- Quezada SA, Simpson TR, Peggs KS, et al. Tumor-reactive CD4(+) T cells develop cytotoxic activity and eradicate large established melanoma after transfer into lymphopenic hosts. J Exp Med 2010;207:637-50. [PubMed]
- Zelba H, Weide B, Martens A, et al. The prognostic impact of specific CD4 T-cell responses is critically dependent on the target antigen in melanoma. Oncoimmunology 2015;4:e955683. [PubMed]
- Zelba H, Weide B, Martens A, et al. Circulating CD4+ T cells that produce IL4 or IL17 when stimulated by melan-A but not by NY-ESO-1 have negative impacts on survival of patients with stage IV melanoma. Clin Cancer Res 2014;20:4390-9. [PubMed]
- Jacobs JF, Nierkens S, Figdor CG, et al. Regulatory T cells in melanoma: the final hurdle towards effective immunotherapy? Lancet Oncol 2012;13:e32-42. [PubMed]
- Zheng Y, Josefowicz SZ, Kas A, et al. Genome-wide analysis of Foxp3 target genes in developing and mature regulatory T cells. Nature 2007;445:936-40. [PubMed]
- Jandus C, Bioley G, Speiser DE, et al. Selective accumulation of differentiated FOXP3(+) CD4 (+) T cells in metastatic tumor lesions from melanoma patients compared to peripheral blood. Cancer Immunol Immunother 2008;57:1795-805. [PubMed]
- Javia LR, Rosenberg SA. CD4+CD25+ suppressor lymphocytes in the circulation of patients immunized against melanoma antigens. J Immunother 2003;26:85-93. [PubMed]
- Viguier M, Lemaître F, Verola O, et al. Foxp3 expressing CD4+CD25(high) regulatory T cells are overrepresented in human metastatic melanoma lymph nodes and inhibit the function of infiltrating T cells. J Immunol 2004;173:1444-53. [PubMed]
- Ahmadzadeh M, Felipe-Silva A, Heemskerk B, et al. FOXP3 expression accurately defines the population of intratumoral regulatory T cells that selectively accumulate in metastatic melanoma lesions. Blood 2008;112:4953-60. [PubMed]
- Curiel TJ, Coukos G, Zou L, et al. Specific recruitment of regulatory T cells in ovarian carcinoma fosters immune privilege and predicts reduced survival. Nat Med 2004;10:942-9. [PubMed]
- Miracco C, Mourmouras V, Biagioli M, et al. Utility of tumour-infiltrating CD25+FOXP3+ regulatory T cell evaluation in predicting local recurrence in vertical growth phase cutaneous melanoma. Oncol Rep 2007;18:1115-22. [PubMed]
- Mourmouras V, Fimiani M, Rubegni P, et al. Evaluation of tumour-infiltrating CD4+CD25+FOXP3+ regulatory T cells in human cutaneous benign and atypical naevi, melanomas and melanoma metastases. Br J Dermatol 2007;157:531-9. [PubMed]
- Hillen F, Baeten CI, van de Winkel A, et al. Leukocyte infiltration and tumor cell plasticity are parameters of aggressiveness in primary cutaneous melanoma. Cancer Immunol Immunother 2008;57:97-106. [PubMed]
- Quezada SA, Peggs KS, Curran MA, et al. CTLA-4 blockade and GM-CSF combination immunotherapy alters the intratumor balance of effector and regulatory T cells. J Clin Invest 2006;116:1935-45. [PubMed]
- Ahmadzadeh M, Rosenberg SA. IL-2 administration increases CD4+ CD25(hi) Foxp3+ regulatory T cells in cancer patients. Blood 2006;107:2409-14. [PubMed]
- Haanen JB, Baars A, Gomez R, et al. Melanoma-specific tumor-infiltrating lymphocytes but not circulating melanoma-specific T cells may predict survival in resected advanced-stage melanoma patients. Cancer Immunol Immunother 2006;55:451-8. [PubMed]
- Ahmadzadeh M, Johnson LA, Heemskerk B, et al. Tumor antigen-specific CD8 T cells infiltrating the tumor express high levels of PD-1 and are functionally impaired. Blood 2009;114:1537-44. [PubMed]
- Zippelius A, Batard P, Rubio-Godoy V, et al. Effector function of human tumor-specific CD8 T cells in melanoma lesions: a state of local functional tolerance. Cancer Res 2004;64:2865-73. [PubMed]
- Baitsch L, Baumgaertner P, Devevre E, et al. Exhaustion of tumor-specific CD8(+) T cells in metastases from melanoma patients. J Clin Invest 2011;121:2350-60. [PubMed]
- Ladányi A, Kiss J, Mohos A, et al. Prognostic impact of B-cell density in cutaneous melanoma. Cancer Immunol Immunother 2011;60:1729-38. [PubMed]
- Martínez-Escribano JA, Hernández-Caselles T, Campillo JA, et al. Changes in the number of CD80(+), CD86(+), and CD28(+) peripheral blood lymphocytes have prognostic value in melanoma patients. Hum Immunol 2003;64:796-801. [PubMed]
- Carpenter EL, Mick R, Rech AJ, et al. Collapse of the CD27+ B-cell compartment associated with systemic plasmacytosis in patients with advanced melanoma and other cancers. Clin Cancer Res 2009;15:4277-87. [PubMed]
- Lauerova L, Dusek L, Simickova M, et al. Malignant melanoma associates with Th1/Th2 imbalance that coincides with disease progression and immunotherapy response. Neoplasma 2002;49:159-66. [PubMed]
- Staquicini FI, Tandle A, Libutti SK, et al. A subset of host B lymphocytes controls melanoma metastasis through a melanoma cell adhesion molecule/MUC18-dependent interaction: evidence from mice and humans. Cancer Res 2008;68:8419-28. [PubMed]
- Kawakami Y, Yaguchi T, Sumimoto H, et al. Improvement of cancer immunotherapy by combining molecular targeted therapy. Front Oncol 2013;3:136. [PubMed]
- Sumimoto H, Imabayashi F, Iwata T, et al. The BRAF-MAPK signaling pathway is essential for cancer-immune evasion in human melanoma cells. J Exp Med 2006;203:1651-6. [PubMed]
- Spranger S, Bao R, Gajewski TF. Melanoma-intrinsic beta-catenin signalling prevents anti-tumour immunity. Nature 2015;523:231-5. [PubMed]
- Dustin ML. The immunological synapse. Cancer Immunol Res 2014;2:1023-33. [PubMed]
- Melero I, Berman DM, Aznar MA, et al. Evolving synergistic combinations of targeted immunotherapies to combat cancer. Nat Rev Cancer 2015;15:457-72. [PubMed]
- Shuford WW, Klussman K, Tritchler DD, et al. 4-1BB costimulatory signals preferentially induce CD8+ T cell proliferation and lead to the amplification in vivo of cytotoxic T cell responses. J Exp Med 1997;186:47-55. [PubMed]
- Wang C, Lin GH, McPherson AJ, et al. Immune regulation by 4-1BB and 4-1BBL: complexities and challenges. Immunological reviews 2009;229:192-215. [PubMed]
- Houot R, Kohrt HE, Marabelle A, et al. Targeting immune effector cells to promote antibody-induced cytotoxicity in cancer immunotherapy. Trends Immunol 2011;32:510-6. [PubMed]
- Hintzen RQ, Lens SM, Beckmann MP, et al. Characterization of the human CD27 ligand, a novel member of the TNF gene family. J Immunol 1994;152:1762-73. [PubMed]
- Vonderheide RH, Glennie MJ. Agonistic CD40 antibodies and cancer therapy. Clin Cancer Res 2013;19:1035-43. [PubMed]
- Schaer DA, Murphy JT, Wolchok JD. Modulation of GITR for cancer immunotherapy. Curr Opin Immunol 2012;24:217-24. [PubMed]
- Ruby CE, Redmond WL, Haley D, et al. Anti-OX40 stimulation in vivo enhances CD8+ memory T cell survival and significantly increases recall responses. Eur J Immunol 2007;37:157-66. [PubMed]
- Leach DR, Krummel MF, Allison JP. Enhancement of antitumor immunity by CTLA-4 blockade. Science 1996;271:1734-6. [PubMed]
- Krummel MF, Allison JP. CD28 and CTLA-4 have opposing effects on the response of T cells to stimulation. J Exp Med 1995;182:459-65. [PubMed]
- Tivol EA, Borriello F, Schweitzer AN, et al. Loss of CTLA-4 leads to massive lymphoproliferation and fatal multiorgan tissue destruction, revealing a critical negative regulatory role of CTLA-4. Immunity 1995;3:541-7. [PubMed]
- Waterhouse P, Penninger JM, Timms E, et al. Lymphoproliferative disorders with early lethality in mice deficient in Ctla-4. Science 1995;270:985-8. [PubMed]
- Kershaw MH, Westwood JA, Darcy PK. Gene-engineered T cells for cancer therapy. Nat Rev Cancer 2013;13:525-41. [PubMed]
- Qureshi OS, Zheng Y, Nakamura K, et al. Trans-endocytosis of CD80 and CD86: a molecular basis for the cell-extrinsic function of CTLA-4. Science 2011;332:600-3. [PubMed]
- Simpson TR, Li F, Montalvo-Ortiz W, et al. Fc-dependent depletion of tumor-infiltrating regulatory T cells co-defines the efficacy of anti-CTLA-4 therapy against melanoma. J Exp Med 2013;210:1695-710. [PubMed]
- Okazaki T, Chikuma S, Iwai Y, et al. A rheostat for immune responses: the unique properties of PD-1 and their advantages for clinical application. Nat Immunol 2013;14:1212-8. [PubMed]
- Sharpe AH, Wherry EJ, Ahmed R, et al. The function of programmed cell death 1 and its ligands in regulating autoimmunity and infection. Nat Immunol 2007;8:239-45. [PubMed]
- Nishimura H, Minato N, Nakano T, et al. Immunological studies on PD-1 deficient mice: implication of PD-1 as a negative regulator for B cell responses. Int Immunol 1998;10:1563-72. [PubMed]
- Blazar BR, Carreno BM, Panoskaltsis-Mortari A, et al. Blockade of programmed death-1 engagement accelerates graft-vs.-host disease lethality by an IFN-gamma-dependent mechanism. J Immunol 2003;171:1272-7. [PubMed]
- Riley JL. PD-1 signaling in primary T cells. Immunol Rev 2009;229:114-25. [PubMed]
- Topalian SL, Drake CG, Pardoll DM. Immune checkpoint blockade: a common denominator approach to cancer therapy. Cancer cell 2015;27:450-61. [PubMed]
- Sierro S, Romero P, Speiser DE. The CD4-like molecule LAG-3, biology and therapeutic applications. Expert Opin Ther Targets 2011;15:91-101. [PubMed]
- Huard B, Gaulard P, Faure F, et al. Cellular expression and tissue distribution of the human LAG-3-encoded protein, an MHC class II ligand. Immunogenetics 1994;39:213-7. [PubMed]
- Baixeras E, Huard B, Miossec C, et al. Characterization of the lymphocyte activation gene 3-encoded protein. A new ligand for human leukocyte antigen class II antigens. J Exp Med 1992;176:327-37. [PubMed]
- Kouo T, Huang L, Pucsek AB, et al. Galectin-3 Shapes Antitumor Immune Responses by Suppressing CD8+ T Cells via LAG-3 and Inhibiting Expansion of Plasmacytoid Dendritic Cells. Cancer Immunol Res 2015;3:412-23. [PubMed]
- Workman CJ, Vignali DA. The CD4-related molecule, LAG-3 (CD223), regulates the expansion of activated T cells. Eur J Immunol 2003;33:970-9. [PubMed]
- Miyazaki T, Dierich A, Benoist C, et al. LAG-3 is not responsible for selecting T helper cells in CD4-deficient mice. Int Immunol 1996;8:725-9. [PubMed]
- Bettini M, Szymczak-Workman AL, Forbes K, et al. Cutting edge: accelerated autoimmune diabetes in the absence of LAG-3. J Immunol 2011;187:3493-8. [PubMed]
- Sánchez-Fueyo A, Tian J, Picarella D, et al. Tim-3 inhibits T helper type 1-mediated auto- and alloimmune responses and promotes immunological tolerance. Nat Immunol 2003;4:1093-101. [PubMed]
- Rangachari M, Zhu C, Sakuishi K, et al. Bat3 promotes T cell responses and autoimmunity by repressing Tim-3-mediated cell death and exhaustion. Nat Med 2012;18:1394-400. [PubMed]
- Zhu C, Anderson AC, Schubart A, et al. The Tim-3 ligand galectin-9 negatively regulates T helper type 1 immunity. Nat Immunol 2005;6:1245-52. [PubMed]
- Huang YH, Zhu C, Kondo Y, et al. CEACAM1 regulates TIM-3-mediated tolerance and exhaustion. Nature 2015;517:386-90. [PubMed]
- Benson DM Jr, Caligiuri MA. Killer immunoglobulin-like receptors and tumor immunity. Cancer Immunol Res 2014;2:99-104. [PubMed]
- Wang L, Rubinstein R, Lines JL, et al. VISTA, a novel mouse Ig superfamily ligand that negatively regulates T cell responses. J Exp Med 2011;208:577-92. [PubMed]
- Le Mercier I, Chen W, Lines JL, et al. VISTA Regulates the Development of Protective Antitumor Immunity. Cancer Res 2014;74:1933-44. [PubMed]
- Suh WK, Gajewska BU, Okada H, et al. The B7 family member B7-H3 preferentially down-regulates T helper type 1-mediated immune responses. Nat Immunol 2003;4:899-906. [PubMed]
- Yi KH, Chen L. Fine tuning the immune response through B7-H3 and B7-H4. Immunol Rev 2009;229:145-51. [PubMed]
- Johnston RJ, Comps-Agrar L, Hackney J, et al. The immunoreceptor TIGIT regulates antitumor and antiviral CD8(+) T cell effector function. Cancer Cell 2014;26:923-37. [PubMed]
- Prendergast GC, Smith C, Thomas S, et al. Indoleamine 2,3-dioxygenase pathways of pathogenic inflammation and immune escape in cancer. Cancer Immunol Immunother 2014;63:721-35. [PubMed]
- Rosenberg SA. IL-2: the first effective immunotherapy for human cancer. J Immunol 2014;192:5451-8. [PubMed]
- Mocellin S, Lens MB, Pasquali S, et al. Interferon alpha for the adjuvant treatment of cutaneous melanoma. Cochrane Database Syst Rev 2013;6:CD008955. [PubMed]
- Ribas A, Puzanov I, Dummer R, et al. Pembrolizumab versus investigator-choice chemotherapy for ipilimumab-refractory melanoma (KEYNOTE-002): a randomised, controlled, phase 2 trial. Lancet Oncol 2015;16:908-18. [PubMed]
- Robert C, Long GV, Brady B, et al. Nivolumab in previously untreated melanoma without BRAF mutation. N Engl J Med 2015;372:320-30. [PubMed]
- Robert C, Schachter J, Long GV, et al. Pembrolizumab vs. Ipilimumab in Advanced Melanoma. N Engl J Med 2015;372:2521-32. [PubMed]
- Weber JS, D'Angelo SP, Minor D, et al. Nivolumab vs. chemotherapy in patients with advanced melanoma who progressed after anti-CTLA-4 treatment (CheckMate 037): a randomised, controlled, open-label, phase 3 trial. Lancet Oncol 2015;16:375-84. [PubMed]
- Perez-Gracia JL, Labiano S, Rodriguez-Ruiz ME, et al. Orchestrating immune check-point blockade for cancer immunotherapy in combinations. Curr Opin Immunol 2014;27:89-97. [PubMed]
- Postow MA, Chesney J, Pavlick AC, et al. Nivolumab and ipilimumab vs. ipilimumab in untreated melanoma. N Engl J Med 2015;372:2006-17. [PubMed]
- Baitsch L, Legat A, Barba L, et al. Extended co-expression of inhibitory receptors by human CD8 T-cells depending on differentiation, antigen-specificity and anatomical localization. PloS One 2012;7:e30852. [PubMed]
- Matsuzaki J, Gnjatic S, Mhawech-Fauceglia P, et al. Tumor-infiltrating NY-ESO-1-specific CD8+ T cells are negatively regulated by LAG-3 and PD-1 in human ovarian cancer. Proc Natl Acad Sci U S A 2010;107:7875-80. [PubMed]
- Romagné F, André P, Spee P, et al. Preclinical characterization of 1-7F9, a novel human anti-KIR receptor therapeutic antibody that augments natural killer-mediated killing of tumor cells. Blood 2009;114:2667-77. [PubMed]
- Loo D, Alderson RF, Chen FZ, et al. Development of an Fc-enhanced anti-B7-H3 monoclonal antibody with potent antitumor activity. Clin Cancer Res 2012;18:3834-45. [PubMed]
- Tirapu I, Mazzolini G, Rodriguez-Calvillo M, et al. Effective tumor immunotherapy: start the engine, release the brakes, step on the gas pedal,and get ready to face autoimmunity. Arch Immunol Ther Exp (Warsz) 2002;50:13-8. [PubMed]
- Sanlorenzo M, Vujic I, Moy A, et al. Synergy of molecular targeted approaches and immunotherapy in melanoma: preclinical basis and clinical perspectives. Expert Opin Biol Ther 2015;15:1491-500. [PubMed]
- Frederick DT, Piris A, Cogdill AP, et al. BRAF inhibition is associated with enhanced melanoma antigen expression and a more favorable tumor microenvironment in patients with metastatic melanoma. Clin Cancer Res 2013;19:1225-31. [PubMed]
- Sapkota B, Hill CE, Pollack BP. Vemurafenib enhances MHC induction in BRAF homozygous melanoma cells. Oncoimmunology 2013;2:e22890. [PubMed]
- Boni A, Cogdill AP, Dang P, et al. Selective BRAFV600E inhibition enhances T-cell recognition of melanoma without affecting lymphocyte function. Cancer Res 2010;70:5213-9. [PubMed]
- Jiang X, Zhou J, Giobbie-Hurder A, et al. The activation of MAPK in melanoma cells resistant to BRAF inhibition promotes PD-L1 expression that is reversible by MEK and PI3K inhibition. Clin Cancer Res 2013;19:598-609. [PubMed]
- Atefi M, Avramis E, Lassen A, et al. Effects of MAPK and PI3K pathways on PD-L1 expression in melanoma. Clin Cancer Res 2014;20:3446-57. [PubMed]
- Wilmott JS, Long GV, Howle JR, et al. Selective BRAF inhibitors induce marked T-cell infiltration into human metastatic melanoma. Clin Cancer Res 2012;18:1386-94. [PubMed]
- Callahan MK, Masters G, Pratilas CA, et al. Paradoxical activation of T cells via augmented ERK signaling mediated by a RAF inhibitor. Cancer Immunol Res 2014;2:70-9. [PubMed]
- Vella LJ, Pasam A, Dimopoulos N, et al. MEK inhibition, alone or in combination with BRAF inhibition, affects multiple functions of isolated normal human lymphocytes and dendritic cells. Cancer Immunol Res 2014;2:351-60. [PubMed]
- Wargo JA, Cooper ZA, Flaherty KT. Universes collide: combining immunotherapy with targeted therapy for cancer. Cancer Discov 2014;4:1377-86. [PubMed]
- Liu L, Mayes PA, Eastman S, et al. The BRAF and MEK Inhibitors Dabrafenib and Trametinib: Effects on Immune Function and in Combination with Immunomodulatory Antibodies Targeting PD-1, PD-L1, and CTLA-4. Clin Cancer Res 2015;21:1639-51. [PubMed]
- Hu-Lieskovan S, Mok S, Homet Moreno B, et al. Improved antitumor activity of immunotherapy with BRAF and MEK inhibitors in BRAF(V600E) melanoma. Sci Transl Med 2015;7:279ra41.
- Ferrari de Andrade L, Ngiow SF, Stannard K, et al. Natural killer cells are essential for the ability of BRAF inhibitors to control BRAFV600E-mutant metastatic melanoma. Cancer Res 2014;74:7298-308. [PubMed]


