Lower body mass index is associated with a higher risk of giant cell arteritis: a systematic review and meta-analysis
Introduction
Giant cell arteritis (GCA) is a form of vasculitis characterized by granulomatous inflammation of medium and large-sized arteries (1). It is the most common vasculitis in Western countries with the highest reported incidence in Scandinavian countries and Minnesota, United States of America. In these populations, with a shared ethnic background, an annual incidence of ~20/100,000 population >50 years of age has been estimated (2,3). GCA typically affects women older than 50 years of age with a peak incidence among those aged 75-85 years (4,5). Patients with GCA usually present with characteristic signs and symptoms of systemic inflammation and cranial ischemia including new-onset headache, visual changes, jaw claudication and scalp tenderness (1,4). GCA is associated with significant morbidity and mortality, including visual loss from ischemic optic neuropathy, cerebrovascular accident, aortic aneurysm and aortic dissection, secondary to the damaged arterial wall and its ischemic complications (1,5).
There are limited data on predictors of GCA. In 2006, Larsson had demonstrated a statistically significant inverse relationship between risk of developing GCA and body mass index (BMI) (6). This raises the question of whether the higher BMI could be a protective factor against GCA. Nevertheless, subsequent epidemiological studies attempting to answer this question yielded inconsistent results (7,8). Thus, to further investigate this possible association, we conducted a systematic review and meta-analysis of epidemiological studies that investigated the relationship between BMI and risk of subsequent development of GCA.
Methods
Search strategy
A comprehensive search was conducted by two investigators (P.U. and C.T.) who independently searched published studies indexed in MEDLINE and EMBASE database from inception to November 2014 using the search terms described in Supplementary data. References of selected articles were also manually searched.
Inclusion criteria
The inclusion criteria were as follows: (I) observational study (case-control or cohort study) published as original study or conference abstract reporting BMI of patients with GCA prior to the diagnosis of GCA compared with subjects without GCA (II) relative risk (RR), odds ratio (OR) or hazard ratio (HR) with 95% confidence interval (CI) from regression analysis were provided.
Study eligibility was independently determined by each investigator noted above. Any disagreements were resolved by with the senior investigator (K.J.W.). Quality of the included cohort and case-control studies was, again, independently evaluated by the two investigators using the Newcastle-Ottawa quality assessment scale (9) which assessed each study with eight items in three areas including (I) the selection of the study groups, (II) the comparability of cases and controls, and (III) the ascertainment of the exposure or the assessment of outcome of interest for case-control or cohort studies respectively.
Exclusion criteria
The exclusion criteria were as follows: (I) interventional study; (II) descriptive study without control group; (III) cross-sectional study as this type of study cannot evaluate the temporal relationship between BMI and risk of GCA.
Data extraction
All investigators independently extracted data from each study using a standardized data collection form. This information was extracted: last name of the first author, title of the study, study design, year of publication, country where the study was conducted, numbers of cases and controls, method used to identify cases and controls, criteria used for the diagnosis of GCA, method used to measure BMI, time when BMI was measured and basic epidemiological data of cases and controls. Any discrepancies in data extraction were resolved by referring back to the original studies.
Statistical analysis
Data analysis was performed using Review Manager 5.3 software from the Cochrane Collaboration. Point estimates and standard errors were extracted from individual studies and were combined by the generic inverse variance method of DerSimonian and Laird (10). HRs and RRs were used as an estimate for OR. Fixed effect model was utilized. Cochran’s Q test was used to determine the statistical heterogeneity of this study. This test was complemented with the I2 statistic, which quantified the proportion of the total variation across studies that is due to heterogeneity rather than chance. A value of I2 of 0% to 25% indicates insignificant heterogeneity, 26% to 50% indicates low heterogeneity, 51% to 75% indicates moderate heterogeneity, and 76% to 100% indicate high heterogeneity (11).
Results
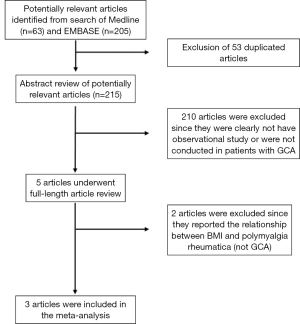
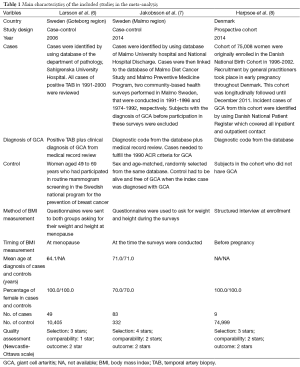
Our search strategy yielded 268 potentially relevant studies (63 articles from Medline and 205 articles from EMBASE). After the exclusion of 53 duplications, 215 articles underwent title and abstract review. Two hundred and ten articles were excluded as they were clearly not observational studies, were not conducted in patients with GCA or did not report patient’s BMI, leaving 5 articles for full-length article review. Two articles were excluded since they evaluated the association between BMI and risk of subsequent development of polymyalgia rheumatica, but not GCA (12,13). Three studies [(two case-control studies (6,7) and one cohort study (8)] with 141 patients with GCA and 85,736 controls met our eligibility criteria were included in our data analyses. Figure 1 outlines the search methodology and review process. The detailed characteristics of the included studies are illustrated in Table 1.
Full table
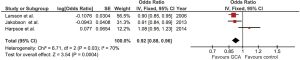
Our study is the first meta-analysis to demonstrate a statistically significant inverse relationship between BMI and risk of subsequent development of GCA as the risk increased by 8% when BMI was reduced by 1.0 kg/m2 (pooled OR of 0.92/kg/m2; 95% CI, 0.88-0.96). The statistical heterogeneity was moderate with an I2 of 70%. Figure 2 demonstrates the forest plots of this meta-analysis.
As the statistical heterogeneity was not low in this meta-analysis, we also performed another analysis using random-effect model which yielded a slightly different pooled estimated effect and a wider 95% CI (pooled OR of 0.94/kg/m2; 95% CI, 0.86-1.03).
As the study by Larsson et al. (6) and Harpsoe et al. (8) included only female subjects while study by Jakobsson et al. (7) was the only that also included male subjects, we performed a sensitivity analysis by using the data of female subjects (instead of all subjects) from Jakobsson’s study for the data analysis. This sensitivity analysis did not significantly alter the results as the pooled OR only slightly decreased to 0.91/kg/m2 with 95% CI of 0.87-0.96.
Evaluation for publication bias
We did not conduct an evaluation for publication bias as only three studies were included in this meta-analysis.
Discussion
A negative correlation between BMI and risk of GCA was demonstrated in this meta-analysis. Risk of subsequent development of GCA was reduced by 8% with every 1 kg/m2 increase in BMI. It is unclear as to why individuals with higher BMI have a lower risk of GCA. There are few potential explanations.
The first hypothesis is related to the relationship between BMI and diabetes mellitus (DM). GCA is an antigen-driven inflammatory process, although the inciting antigen(s) have yet to be identified (14). These inciting antigen(s) are recognized by dendritic cells residing in the arterial wall, which then initiate the inflammatory cascade by recruiting T cells and macrophages to form granulomas (15-17). Patients with DM may have a lower likelihood of developing GCA as a result of the decreased responsiveness of dendritic cells and T cells to the putative antigen(s) secondary to hyperglycemic state (18-21). In fact, several epidemiological studies have demonstrated a lower prevalence of DM in patients with GCA compared with sex and age-matched controls (22-27). It is well documented that individuals with higher BMI have a higher risk of the development of DM (28-30) and this could be responsible for their lower risk of GCA.
The second possible hypothesis is related to the anti-inflammatory property of female sex hormones (31-33) as estrone, the principal estrogen in male and postmenopausal female, is formed primarily by the conversion of adrenal androstenedione in adipose tissue (34). Moreover, estrogen has been shown to augment the responsiveness of hypothalamo-pituitary-adrenal axis to inflammatory stimuli (35,36). Higher endogenous estrone production in individuals with a higher BMI, which implies a higher amount of total body adipose tissue, could have anti-inflammatory effects and, thus, prevent autoimmune disorders such as GCA.
The major strength of this meta-analysis is the quality of the included studies and the ability to demonstrate the temporal relationship between BMI and GCA as all three studies measured their cohort’s BMI before the onset of GCA. Nonetheless, there are several limitations and, thus, our results should be interpreted with caution.
First, all of the included studies used medical registry-based database to identify GCA cases, raising a concern of coding inaccuracy and incompleteness. Second, statistical heterogeneity was present in this study. Third, two out of three studies exclusively recruited only female patients. This might jeopardize the generalizability of our results, particularly for male patients. Fourth, we cannot perform the evaluation for publication bias and, thus, publication bias might be present. Fifth, there was a big difference between the numbers of cases and controls which might affect the validity of the comparison. Moreover, this is a meta-analysis of observational studies which, at the best, can only demonstrate an association, not causality. Therefore, we cannot make a conclusion that BMI itself versus other potential confounders reduce the GCA risk. For example, smoking is associated with a lower BMI (37,38) and has also been shown to be a strong risk factor for GCA (39,40).
Conclusions
In conclusion, our meta-analysis demonstrated a statistically significant inverse relationship between BMI and risk of subsequent development GCA. The risk increased by 8% when BMI was reduced by 1.0 kg/m2. Our findings should be considered hypothesis-generating and further research is required.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Supplementary data
Search strategy: Ovid Medline
- exp Polymyalgia Rheumatica/
- polymyalgia rheumatica.mp.
- exp Giant Cell Arteritis/
- giant cell arteritis.mp.
- temporal arteritis.mp.
- temporal arterit$.mp.
- cranial arterit$.mp.
- horton disease$.mp.
- pmr.mp.
- or/1-9
- Cohort Studies/
- comparative study/
- follow-up studies/
- prospective studies/
- risk factors/
- cohort.mp.
- groups.mp.
- multivariate.mp.
- compared.mp.
- or/11-19
- body mass index.mp. or exp Body Mass Index/
- body weight.mp. or exp Body Weight/
- obesity.mp. or exp Obesity/
- or/21-23
- 10 and 20 and 24
Search strategy: EMBASE
- body mass index.mp. or exp body mass/
- body weight.mp. or exp body weight/
- obesity.mp. or exp obesity/
- or/1-3
- clinical article/
- controlled study/
- major clinical study/
- prospective study.mp. or exp prospective study/
- cohort.mp.
- compared.mp.
- groups.mp.
- multivariate.mp.
- or/5-12
- polymyalgia rheumatica.mp. or exp rheumatic polymyalgia/
- giant cell arteritis.mp. or exp giant cell arteritis/
- temporal arteritis.mp. or exp temporal arteritis/
- cranial arterit$.mp.
- horton disease$.mp.
- pmr.mp.
- or/14-19
- 4 and 13 and 20
References
- Salvarani C, Cantini F, Hunder GG. Polymyalgia rheumatica and giant-cell arteritis. Lancet 2008;372:234-45. [PubMed]
- Salvarani C, Crowson CS, O'Fallon WM, et al. Reappraisal of the epidemiology of giant cell arteritis in Olmsted County, Minnesota, over a fifty-year period. Arthritis Rheum 2004;51:264-8. [PubMed]
- Nordborg E, Nordborg C. Giant cell arteritis: epidemiological clues to its pathogenesis and an update on its treatment. Rheumatology (Oxford) 2003;42:413-21. [PubMed]
- Gonzalez-Gay MA, Vazquez-Rodriguez TR, Lopez-Diaz MJ, et al. Epidemiology of giant cell arteritis and polymyalgia rheumatica. Arthritis Rheum 2009;61:1454-61. [PubMed]
- Hunder GG. Epidemiology of giant-cell arteritis. Cleve Clin J Med 2002;69 Suppl 2:SII79-82. [PubMed]
- Larsson K, Mellström D, Nordborg E, et al. Early menopause, low body mass index, and smoking are independent risk factors for developing giant cell arteritis. Ann Rheum Dis 2006;65:529-32. [PubMed]
- Jakobsson K, Jacobsson L, Warrington K, et al. Body mass index and the risk of giant cell arteritis: results from a prospective study. Rheumatology (Oxford) 2015;54:433-40. [PubMed]
- Harpsøe MC, Basit S, Andersson M, et al. Body mass index and risk of autoimmune diseases: a study within the Danish National Birth Cohort. Int J Epidemiol 2014;43:843-55. [PubMed]
- Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol 2010;25:603-5. [PubMed]
- DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials 1986;7:177-88. [PubMed]
- Higgins JP, Thompson SG, Deeks JJ, et al. Measuring inconsistency in meta-analyses. BMJ 2003;327:557-60. [PubMed]
- Hoganson DD, Crowson CS, Warrington KJ, et al. Lack of association of high body mass index with risk for developing polymyalgia rheumatica. Int J Rheum Dis 2010;13:e1-5. [PubMed]
- Hemminki K, Li X, Sundquist J, Sundquist K. Risk of asthma and autoimmune diseases and related conditions in patients hospitalized for obesity. Ann Med 2012;44:289-95. [PubMed]
- Weyand CM, Ma-Krupa W, Goronzy JJ. Immunopathways in giant cell arteritis and polymyalgia rheumatica. Autoimmun Rev 2004;3:46-53. [PubMed]
- Weyand CM, Goronzy JJ. Clinical practice. Giant-cell arteritis and polymyalgia rheumatica. N Engl J Med 2014;371:50-7. [PubMed]
- Pryshchep O, Ma-Krupa W, Younge BR, et al. Vessel-specific Toll-like receptor profiles in human medium and large arteries. Circulation 2008;118:1276-84. [PubMed]
- Ly KH, Régent A, Tamby MC, et al. Pathogenesis of giant cell arteritis: More than just an inflammatory condition? Autoimmun Rev 2010;9:635-45. [PubMed]
- Casey J, Sturm C Jr. Impaired response of lymphocytes from non-insulin-dependent diabetics to staphage lysate and tetanus antigen. J Clin Microbiol 1982;15:109-14. [PubMed]
- Casey JI, Heeter BJ, Klyshevich KA. Impaired response of lymphocytes of diabetic subjects to antigen of Staphylococcus aureus. J Infect Dis 1977;136:495-501. [PubMed]
- MacCuish AC, Urbaniak SJ, Campbell CJ, et al. Phytohemagglutinin transformation and circulating lymphocyte subpopulations in insulin-dependent diabetic patients. Diabetes 1974;23:708-12. [PubMed]
- Speert DP, Silva J Jr. Abnormalities of in vitro lymphocyte response to mitogens in diabetic children during acute ketoacidosis. Am J Dis Child 1978;132:1014-7. [PubMed]
- Le Page L, Duhaut P, Seydoux D, et al. Incidence of cardiovascular events in giant cell arteritis: preliminary results of a prospective double cohort study (GRACG). Rev Med Interne 2006;27:98-105. [PubMed]
- Gonzalez-Juanatey C, Lopez-Diaz MJ, Martin J, et al. Atherosclerosis in patients with biopsy-proven giant cell arteritis. Arthritis Rheum 2007;57:1481-6. [PubMed]
- Matthews JL, Gilbert DN, Farris BK, et al. Prevalence of diabetes mellitus in biopsy-positive giant cell arteritis. J Neuroophthalmol 2012;32:202-6. [PubMed]
- Schmidt J, Kermani TA, Muratore F, et al. Statin use in giant cell arteritis: a retrospective study. J Rheumatol 2013;40:910-5. [PubMed]
- Udayakumar PD, Chandran AK, Crowson CS, et al. Cardiovascular risk and acute coronary syndrome in giant cell arteritis: a population-based retrospective cohort study. Arthritis Care Res (Hoboken) 2015;67:396-402. [PubMed]
- Ungprasert P, Koster MJ, Warrington KJ. Coronary artery disease in giant cell arteritis: a systematic review and meta-analysis. Semin Arthritis Rheum 2015;44:586-91. [PubMed]
- Golay A, Ybarra J. Link between obesity and type 2 diabetes. Best Pract Res Clin Endocrinol Metab 2005;19:649-63. [PubMed]
- Faeh D, William J, Tappy L, et al. Prevalence, awareness and control of diabetes in the Seychelles and relationship with excess body weight. BMC Public Health 2007;7:163. [PubMed]
- da Silva RC, Miranda WL, Chacra AR, et al. Insulin resistance, beta-cell function, and glucose tolerance in Brazilian adolescents with obesity or risk factors for type 2 diabetes mellitus. J Diabetes Complications 2007;21:84-92. [PubMed]
- Monteiro R, Teixeira D, Calhau C. Estrogen signaling in metabolic inflammation. Mediators Inflamm 2014;2014:615917.
- Ghisletti S, Meda C, Maggi A, et al. 17beta-estradiol inhibits inflammatory gene expression by controlling NF-kappaB intracellular localization. Mol Cell Biol 2005;25:2957-68. [PubMed]
- Turgeon JL, Carr MC, Maki PM, et al. Complex actions of sex steroids in adipose tissue, the cardiovascular system, and brain: Insights from basic science and clinical studies. Endocr Rev 2006;27:575-605. [PubMed]
- Purohit A, Reed MJ. Regulation of estrogen synthesis in postmenopausal women. Steroids 2002;67:979-83. [PubMed]
- De Leo V, la Marca A, Talluri B, et al. Hypothalamo-pituitary-adrenal axis and adrenal function before and after ovariectomy in premenopausal women. Eur J Endocrinol 1998;138:430-5. [PubMed]
- Giussani DA, Farber DM, Jenkins SL, et al. Opposing effects of androgen and estrogen on pituitary-adrenal function in nonpregnant primates. Biol Reprod. 2000;62:1445-51. [PubMed]
- Akbartabartoori M, Lean ME, Hankey CR. Relationships between cigarette smoking, body size and body shape. Int J Obes (Lond) 2005;29:236-43. [PubMed]
- Eisen SA, Lyons MJ, Goldberg J, et al. The impact of cigarette and alcohol consumption on weight and obesity. An analysis of 1911 monozygotic male twin pairs. Arch Intern Med 1993;153:2457-63. [PubMed]
- Machado EB, Gabriel SE, Beard CM, et al. A population-based case-control study of temporal arteritis: evidence for an association between temporal arteritis and degenerative vascular disease? Int J Epidemiol 1989;18:836-41. [PubMed]
- Duhaut P, Pinede L, Demolombe-Rague S, et al. Giant cell arteritis and cardiovascular risk factors: a multicenter, prospective case-control study. Groupe de Recherche sur l'Artérite à Cellules Géantes. Arthritis Rheum 1998;41:1960-5. [PubMed]